Part of the Catapult Network
Established by Innovate UK

Independent, Not-for-Profit Organisation
Pioneering the next generation of approaches and technologies

Industrialising and Driving Adoption
Of new scientific tools and modern techniques for discovering medicines
Supporting the Drug Discovery Industry
SMEs and innovators to deliver growth for the drug discovery economy
Leveraging UK Infrastructure
Connecting the community
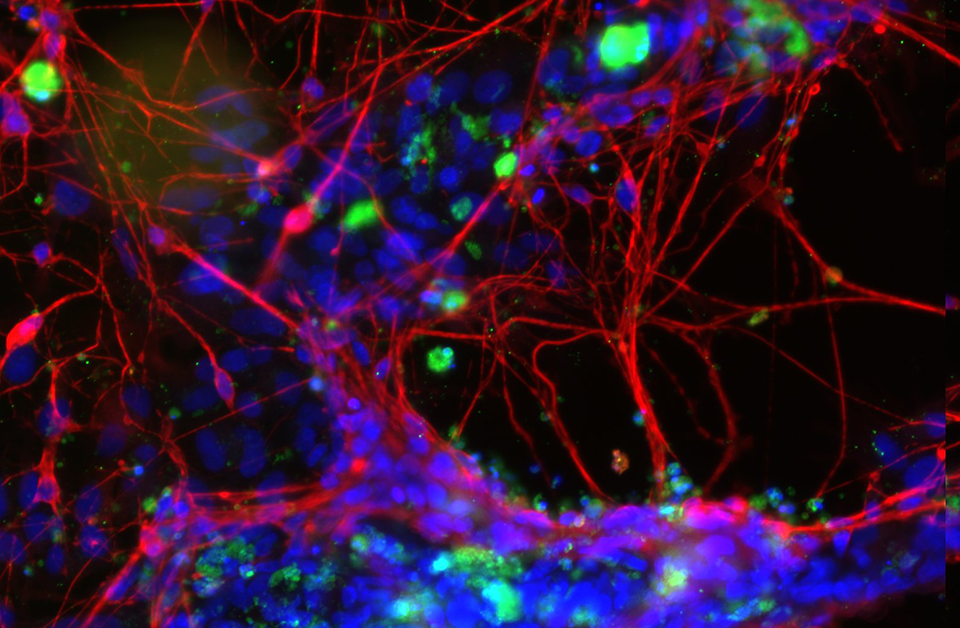
Tackling Industry-led Challenges
That limit today’s discovery process
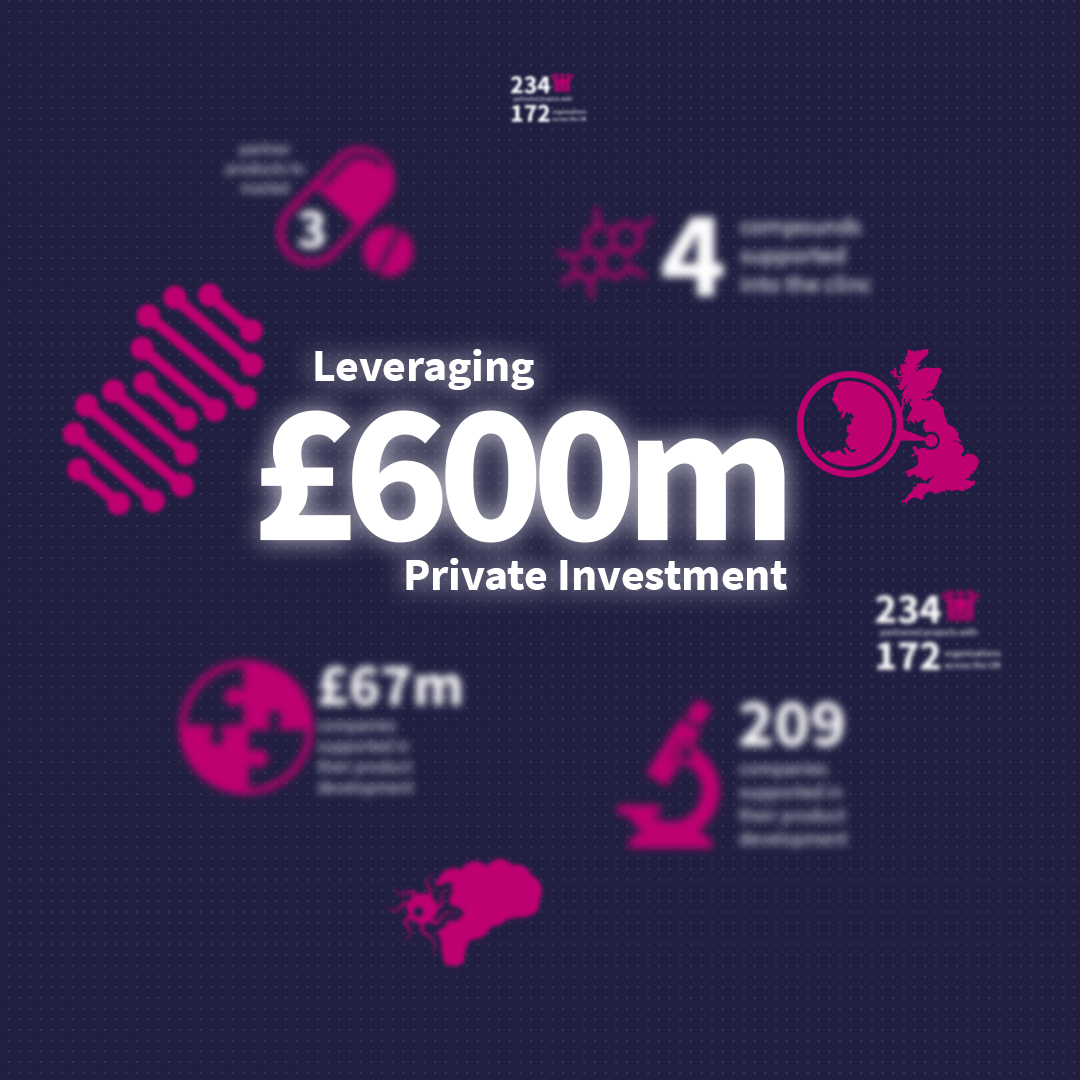
Our Impact
See the impact Medicines Discovery Catapult is having in drug discovery, as we work to reshape drug discovery for patient benefit.
Reshaping Medicines Discovery...
It's ambitious, it's achievable
-
By tapping into MDC’s unique drug development expertise and facilities, we are confident we will accelerate the preclinical development of our drug delivery system and its commercialisation.
Dr Anna Perdrix Rosell
Co-founder and Managing Director, Sixfold Bioscience
-
For early-stage SMEs in this space, I thoroughly recommend engaging with MDC who have the end-to-end knowledge in complex medicines, can support your in-house team and provide access to key equipment that start-ups may not have.
Professor Helen McCarthy
Chief Executive Officer, pHion Therapeutics
-
Working with MDC has provided expertise and intellectual input. It has also enabled access to a wider network of companies providing specialist services.
Dr David Templeton
Technical Director, N4 Pharma
-
As an SME, working with MDC allows us access to scientific know-how and in vivo imaging techniques that would be impossible through any other route. The closeness, timeliness and flexibility of the collaboration significantly accelerates our ability to develop product. MDC perform an absolutely vital role in supporting us getting our novel treatments to clinical trials.
Dr Gareth Wakefield
CTO, Xerion Healthcare Ltd
-
It has been great to access the Artificial Intelligence expertise at Medicines Discovery Catapult, the team have taken an innovative approach that adds value to our product and will benefit our customers. Our collaboration was easy to establish and worked extremely well.
Phil Jones
CSO, BioAscent
-
It has been a pleasure working with MDC as their scientists are pleasant and have a broad range of talents. Importantly for projects like ours, they openly and professionally exchange ideas with SMi’s scientific team and keep us abreast of their research developments. Not surprisingly, we are therefore looking forward to continuing and growing our close partnerships for the foreseeable future.
Dr Andy Thompson
CEO, SMi Systems
-
RevoloBio have had a collaboration with MDC for three years, which continues to provide valuable and high-quality data on our molecules. The input from MDC has directly impacted operational and strategic decisions for our projects and allowed us to move forward with our clinical studies. The MDC team are extraordinarily competent and use a wide range of up-to-date technologies. It has been a seamless and effective collaboration that we will continue.
Dr Roly Foulkes
Chief Scientific Officer, Revolo Biotherapeutics
-
The collaborative partnership with MDC has enabled us to work together to secure multiple Innovate UK grants. MDC has supported Alchemab in developing a therapeutic for Huntington’s Disease through antibody characterisation, iPSC model development, biodistribution studies, innovative imaging and their general neurodegenerative expertise. The partnership has allowed us to utilise capabilities at MDC, such as state-of-the-art PET imaging abilities, which aren’t available elsewhere.
Alchemab Therapeutics
How We Work
SUPPORTING UK INNOVATORS
We are championing UK innovators to succeed in advancing drug discovery.
INDUSTRIALISING NEW TECHNOLOGIES
We help to industrialise and drive the adoption of new technologies and techniques.
COMMUNITY DRIVEN
We are driven by helping our community make their mark on the industry and patients.
All
Championing innovative life science technology and new approaches, supporting UK innovators to succeed.
Loading...