Case Study: Comparative Efficacy of Pluvicto® and PSMA-I&T in PSMA+ PDX Models
July 2025

As radiopharmaceuticals become a central component in treating metastatic castration-resistant prostate cancer (mCRPC), the need for preclinical models that accurately reflect human disease is critical. mCRPC represents an advanced stage of prostate cancer that no longer responds to conventional androgen deprivation therapy, and it is often characterised by widespread metastases, particularly to bone and lymph nodes. Radiopharmaceuticals, such as PSMA-targeted agents, deliver cytotoxic radiation directly to tumor cells by binding to specific molecular targets, offering a promising therapeutic avenue with improved specificity and reduced systemic toxicity. The development of these agents requires extensive radiochemistry expertise and robust preclinical models that can faithfully replicate the complexity of human tumors, including their molecular heterogeneity and treatment resistance mechanisms.
Champions Oncology and Medicines Discovery Catapult (MDC) address this gap by working together to provide an advanced platform that integrates patient-derived xenograft (PDX) models with radiochemistry capabilities. PDX models are established by implanting human tumor tissues directly into immunodeficient mice, preserving the histological architecture, genetic profile, and phenotypic behavior of the original patient tumors. When combined with radiochemistry workflows, including radiolabeling, biodistribution studies, and dosimetry, this platform enables early, data-driven decision-making in radiopharmaceutical development.
Study Overview
In this study, Champions Oncology and Medicines Discovery Catapult conducted a head-to-head comparison of Pluvicto® (Lu-177-PSMA-617) and PSMA I&T radiopharmaceuticals in a clinically annotated, PSMA-positive mCRPC PDX model (CTG-2427). The goal was to evaluate tumor response over time, assess comparative efficacy, and support radiopharmaceutical selection based on translatable in vivo data. CTG-2427 was selected among a panel of prostate cancer PDXs upon verification of PSMA expression by IHC (Figure 1A and B). Both Pluvicto and PSMA I&T were successfully labelled with Lu-177, with a radiochemical purity of >96% and >99% respectfully. The compounds stability was also evaluated over a 24h period and both targeted agents show high stability overtime, as shown in Figure 1C.
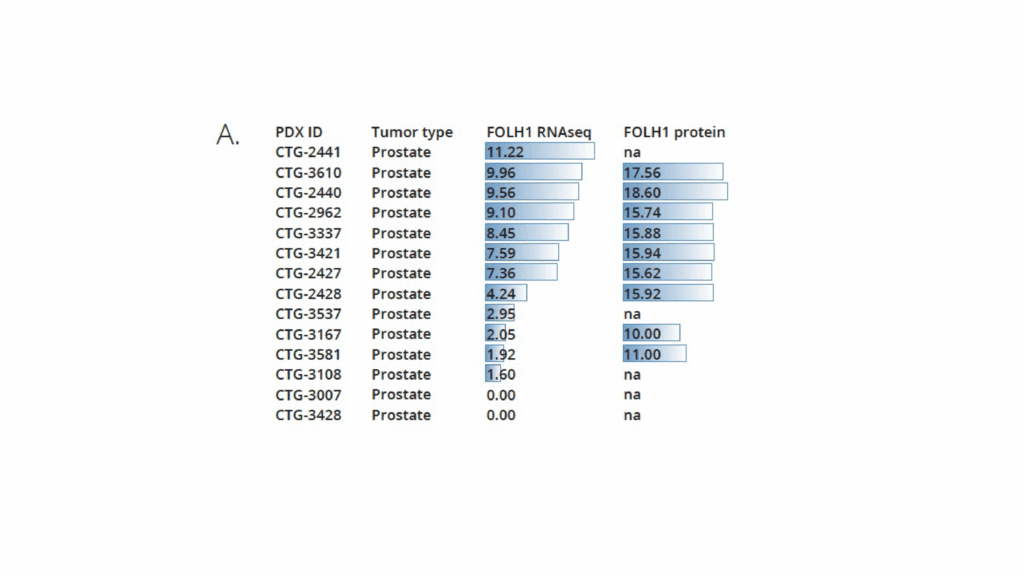
Figure 1. A: PSMA expression in Champions’ prostate cancer PDXs by RNAseq and proteomic analysis.
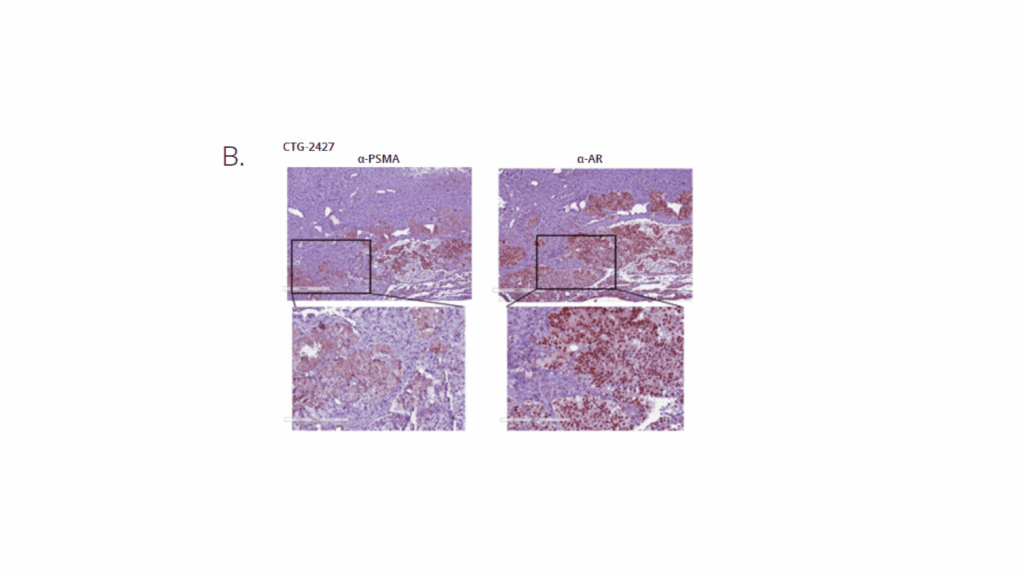
Figure B: PSMA and AR expression and localization in CTG-2427 PDX by IHC.
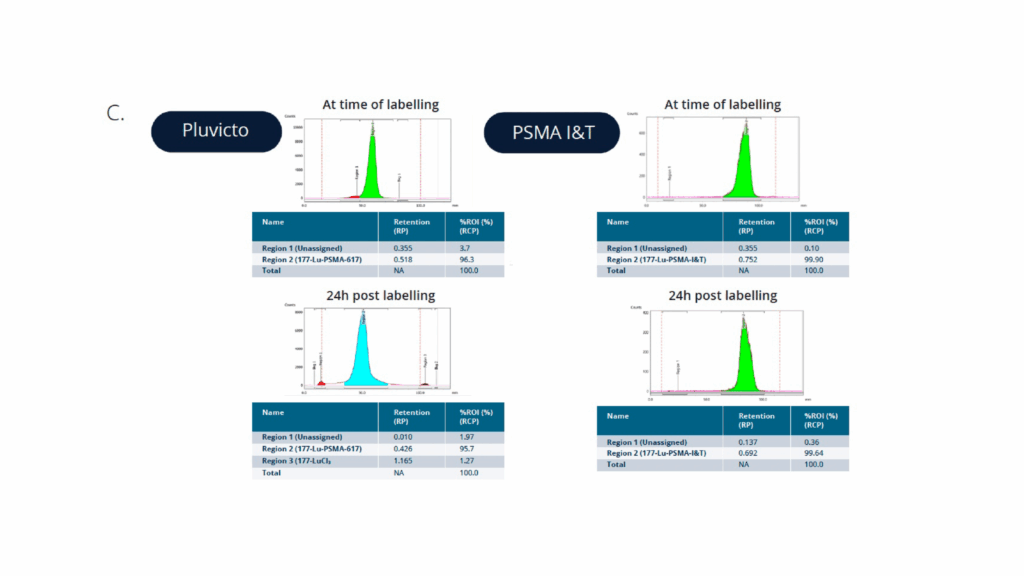
Figure C. Pluvicto® and PSMA-I&T labelling and stability over time.
The results of the in vivo study are shown in Figure 2:
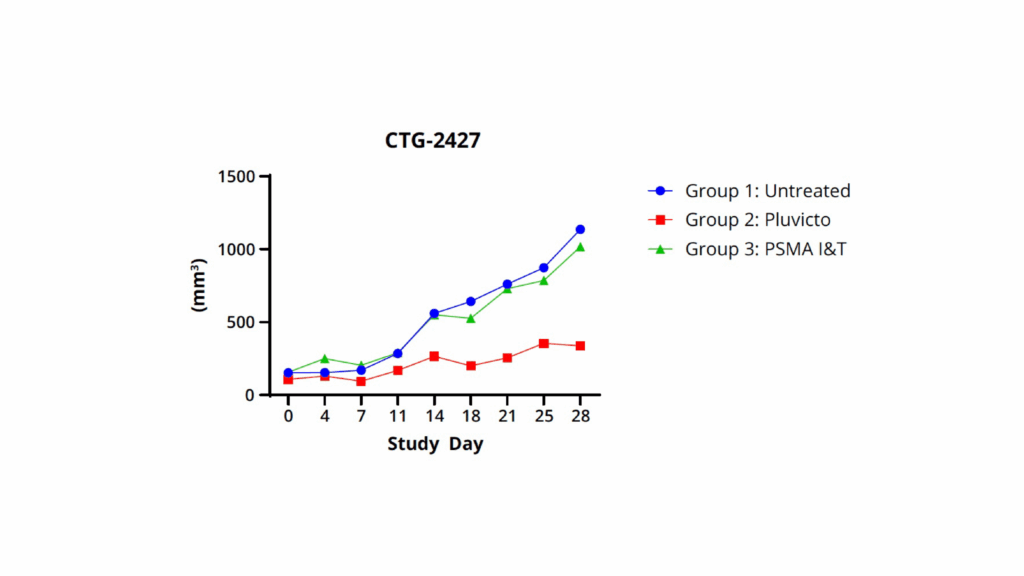
Figure 2: CTG-2427 mice were divided into three groups: Group 1 was untreaded, Group 2 was dosed i.v. with Pluvicto (37 MBq on Day 0 and 18.8 MBq on day 13th) and Group 3 was dosed i.v. with PSMA I&T (37 MBq on Day 0 and 18.8 MBq on day 13th).
The study yielded several significant findings:
The use of PDX models in radiopharmaceutical development offers a strategic advantage by:
PDX models provide a robust platform for the evaluation and development of radiopharmaceuticals, ultimately contributing to improved therapeutic outcomes for patients with mCRPC.
This study highlights the strategic value of using PDX models to benchmark radiopharmaceutical candidates. By mirroring clinical heterogeneity and providing mechanistic insight, PDX-based platforms increase confidence in candidate selection and accelerate the transition from discovery to the clinic.
PDXs enable detailed mechanistic studies that can uncover the underlying biological processes driving treatment responses. By integrating advanced molecular techniques such as RNA sequencing, proteomics, and imaging, researchers can gain insights into the pathways and biomarkers associated with therapeutic efficacy and resistance. This information is crucial for optimizing radiopharmaceutical design, selecting the most promising candidates, and tailoring treatments to individual patient profiles.
The ability to benchmark radiopharmaceuticals in PDXs also facilitates the identification of potential side effects and toxicities early in the development process. This proactive approach helps to mitigate risks and improve the safety profile of new treatments before they reach clinical trials.
Overall, the use of PDXs in radiopharmaceutical development represents a strategic advantage that enhances the reliability of preclinical testing, supports data-driven decision-making, and accelerates the translation of innovative therapies from the laboratory to the clinic.
Preclinical to clinical, building a translational link between in vitro and in vivo models of neuroinflammation for drug discovery.