
BioAscent, a science-led integrated drug discovery: medicinal and synthetic chemistry, computational chemistry, in vitro biosciences, DMPK, compound management and diversity and fragment libraries.
A contract research organisation (CRO) can give flexible access to the right drug discovery capabilities and expertise – the ‘right’ CRO input is closely tailored to your specific drug discovery journey and aims.
Equally, there will be similarities in the challenges you face and those of companies that have trodden a similar path previously. For this reason it is good to partner with a CRO that has worked on comparable projects, one that has helped solve problems you are likely to encounter.
Ultimately, the right CRO can help you rapidly progress your project. They will have the right team in place, and they will definitely ‘get’ what it is you are striving to achieve. It is often the case that you will know whether you are in the right company when you meet a prospective CRO team. Ideally, when you talk with their scientists it will become clear that they can be a great fit for your drug discovery project.
From the CRO’s perspective, it is all about the people. A CRO’s primary asset alongside the physical factors of labs or equipment is their scientists, their expertise and track record of success.
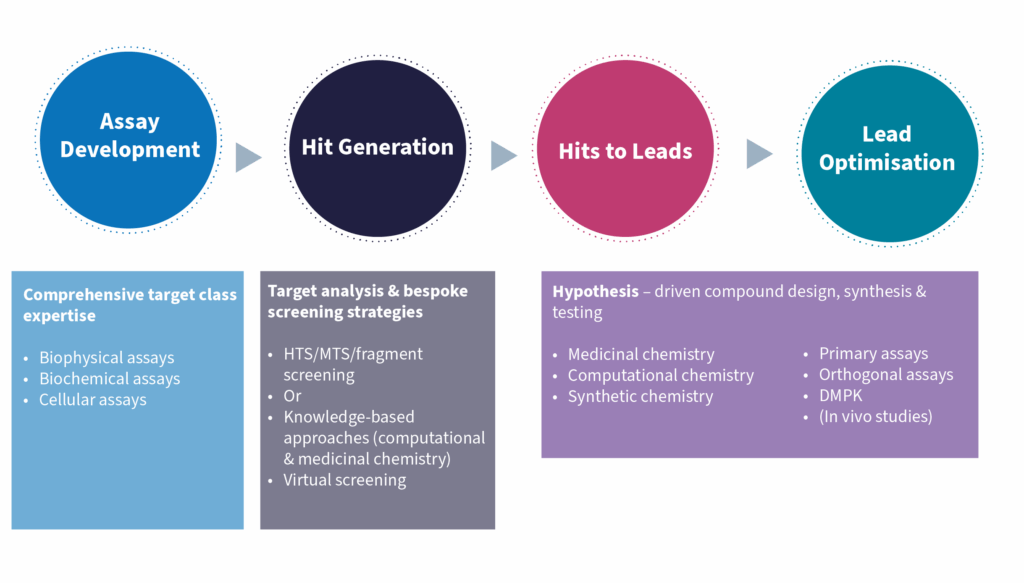
Rapidly Access the Expertise You Need Whatever the stage of a small molecule drug discovery project, a need for additional expertise along the way is almost always present. Biosciences, medicinal and computational chemistry, drug metabolism and pharmacokinetics will likely feature, as will supporting compound management and logistics.
The process begins by establishing exactly what it is your project needs:
Selecting promising CRO candidates can be a time-consuming task, because the sector is large and full of diverse specialisms. There are a variety of ways to make the process easier and quicker:
Initial due diligence should include contacting your most promising candidates, to discover:
With the field narrowed down, a subsequent bidding process can reveal more than just facts and figures.
If the CRO is difficult to get information from, if their programme does not seem closely tailored to your spec and if you suspect they are simply interested in selling you a few ‘pairs of hands’, that CRO is probably not what you need.
The quality – or otherwise – of the scientific input delivered in the bid can also be a useful indicator of the CRO’s actual interest in your project.
Ultimately, the price is obviously a powerful deciding factor.
All of the above are valid discriminators in making the right choice.
Additionally, it is also important to be assured that the scientists you speak with initially are those who will be involved with the project moving forward. It is not unknown for the senior scientists you first encounter to be replaced by the ‘B team’ when work commences.
Customer references can also be very helpful in informing your decision, so these are always worth asking for.
If there is one guiding principle to remember, it is that you should talk to at least two or three of your most preferred CRO options – it is generally the case that you will know the right people when you interact with them.
CRO BioAscent recently worked with Boston-based Arkuda Therapeutics on their project to discover novel Transient Receptor Potential Mucolipin 1(TRPML1) agonists.
TRPML1 is an ion channel found within lysosomes. Mutations and dysfunction of this channel are associated with several conditions, including neurodegenerative diseases such as Alzheimer’s, Amyotrophic Lateral Sclerosis (ALS) and Parkinson’s.
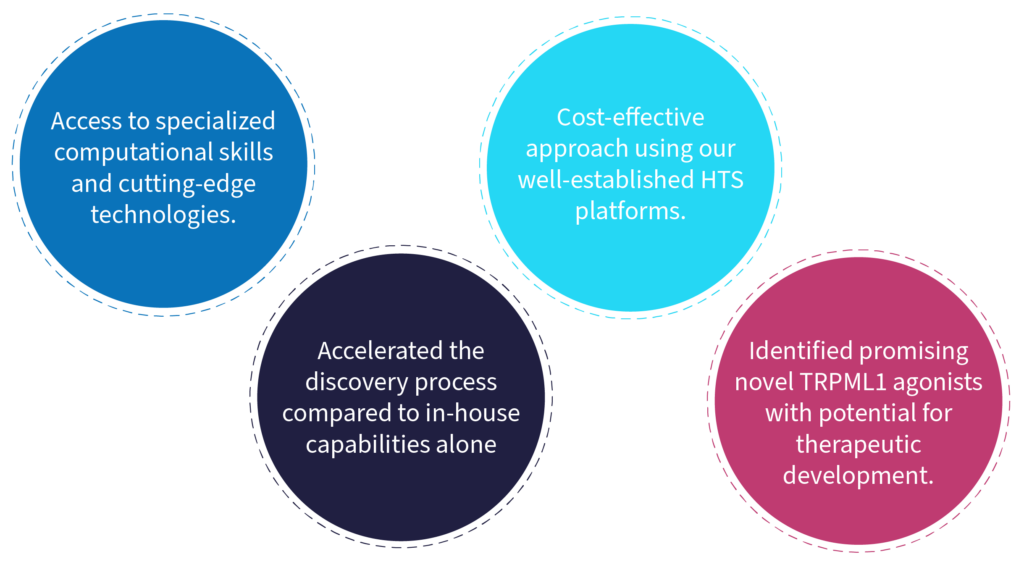
Advantages for Arkuda
Arkuda needed to discover novel small molecule TRPML1 agonists, with a resultant requirement for expertise in hit-finding technologies, specialised skills and resources such as structure-based drug discovery (SBDD) and computer-aided drug design (CADD).
BioAscent with its proven track record in drug discovery provided an ideal fit. The CRO offered high-throughput screening (HTS) of compounds, utilising their own 100,000-plus library of IP-free assets. Highly experienced biologist and in-silico teams also brought their expertise to bear on the project.
For BioAscent this project was characterised by three distinct stages:
Following BioAscent’s Fluorometric Imaging Plate Reader (FLIPR) assay, one compound that ticked many boxes was discovered; a selective and CNS penetrant TRPML1 agonist – shown bottom left below, numbered 1.
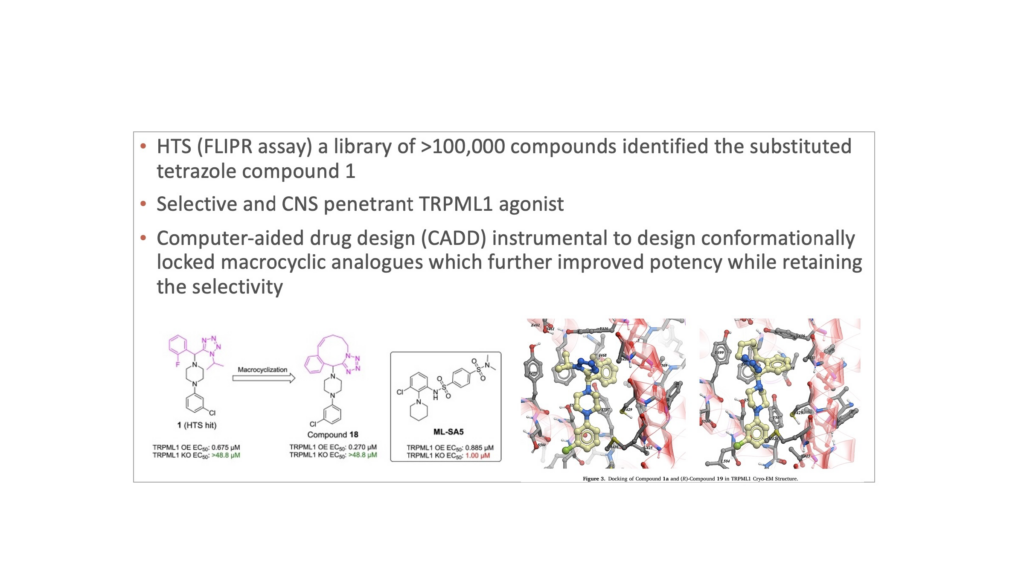
CADD was then used to design conformationally-locked macrocylic analogues, to further improve potency while maintaining selectivity and CNS penetration – compound 18 in the diagram.
Dr Mike Piper Chief Commercial Officer and Dr Angelo Pugliese, Head of In-silico Discovery, BioAscent