Accelerating analgesic development through human-specific in vitro systems
September, 2025

“Medicines Discovery Catapult and ApconiX have collaborated to help de-risk pain therapeutic development through human-specific in vitro models.
Whether you are an SME developing first-in-class non-opioid analgesics or a pharmaceutical company seeking to de-risk a candidate before Phase I, read on to learn more about how we can make your next move count with decision-making data for developing novel pain therapeutics.”
Dr Eve Corrie, Senior Scientist, Medicines Discovery Catapult

“It has been an absolute pleasure collaborating with MDC, applying our complementary technical expertise to validate the utility of hiPSC technology and develop a translatable assay for screening novel peripheral analgesics”
Emily Johnson, ApconiX

It is estimated that as many as half of the UK population live with chronic pain. Considering this figure is likely to increase, and with pain being the common factor in so many different diseases and conditions, the therapies available are very limited.
The development of new treatments for pain has, until recently, felt stalled due to:
However, recently that seems to be changing. In the last year Vertex has gained approval for its NaV1.8 blocker – the first new generalised pain treatment approved in over 20 years. To maintain this progress, we need translational models that better mirror human pain biology.
With this renewed interest in the development of new pain therapies, it has been important to realise that the human pain system is very different to that of the rodent pain system. There is also a shift occurring towards the regulatory acceptance of new approach methodologies (NAMs) for medicines testing and away from the animal safety studies that are traditionally required.
In vitro models that are representative of the human sensory system are therefore a vital tool in the development of new analgesics. Human induced pluripotent stem cell (iPSC) technology offers a way to generate a wide range of human cell types and are is a powerful tool for modelling complex biological systems. These models are increasingly being used to identify and evaluate novel therapeutic strategies in drug discovery.
For this work, RealDRG™ neurons were sourced from Anatomic Incorporated, a provider of cryopreserved, differentiated human iPSCs. RealDRG™ are a model of the sensory neurons of the dorsal root ganglia (DRG), the neurons that are responsible for sensing and transmitting pain signals from the periphery to the central nervous system.
Validation of in vitro cell model systems is essential to demonstrate their translational value and is critical to their successful implementation in the preclinical characterisation workflow.
In this dataset MDC and ApconiX have characterised iPSC-derived sensory neurons for their ability to replicate sensory neurons of the DRG, with the aim to demonstrate and their suitability as a platform for novel therapeutic development.
To ensure the sensory neurons demonstrated a mature phenotype, the cells were immunocytochemically labelled with well-characterised markers of peripheral sensory neurons. They showed strong expression of the voltage gated sodium channel NaV1.8 and the nociceptor-specific marker peripherin. A subpopulation of neurons also strongly labelled for the nociceptive neuropeptide calcitonin gene-related peptide (CGRP), which is also expressed in a subpopulation of neurons in the human DRG. CGRP presence in the lysate of the neurons was further confirmed by a CGRP enzyme-linked immunosorbent assay. These data demonstrate that the cells have the appropriate phenotype to measure physiologically relevant cell behaviour.
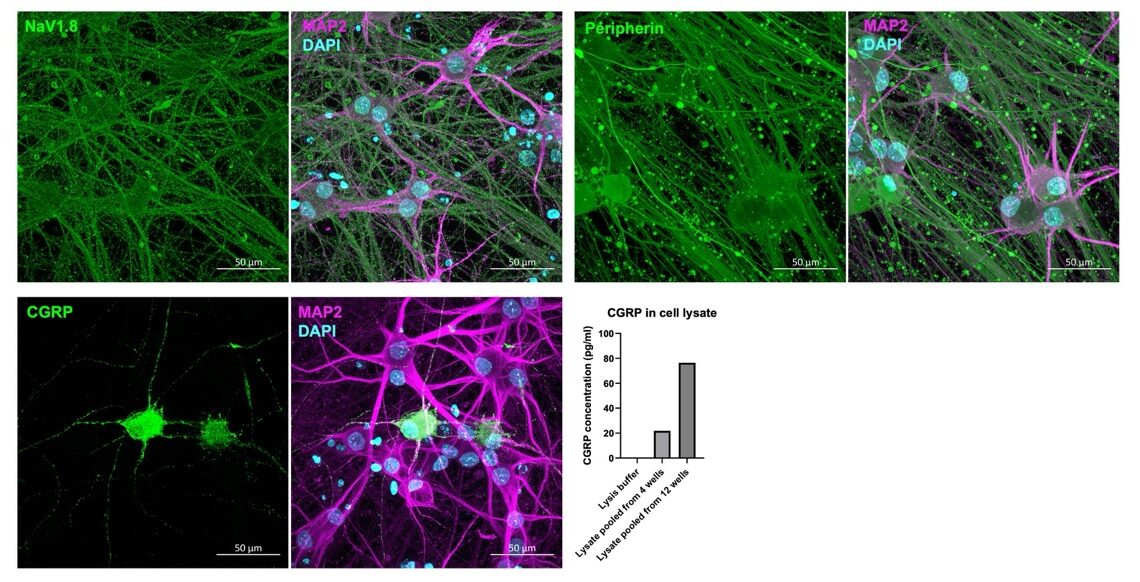
iPSC sensory neurons express key markers of peripheral DRG neurons: Immunocytochemistry and ELISA demonstrate expression of NaV1.8, peripherin and CGRP.
Manual patch clamp demonstrated mature neuronal phenotypes evidenced by repetitive action potential firing upon current injection by 21 days in vitro, as well as typical pseudo-unipolar morphology upon intracellular dye injection. This is a characteristic morphology of DRG neurons in the body, with one long peripheral axon and one long central axon. Neurons with a tetrodotoxin (TTx)-insensitive sodium current component were observed using manual patch clamp. This TTx-insensitive component was further blocked by suzetrigine, a specific NaV1.8 blocker. The presence of a TTx-insensitive sodium current, such as those mediated by Nav1.8, is a key indicator of nociceptor-like function in sensory neuron models.
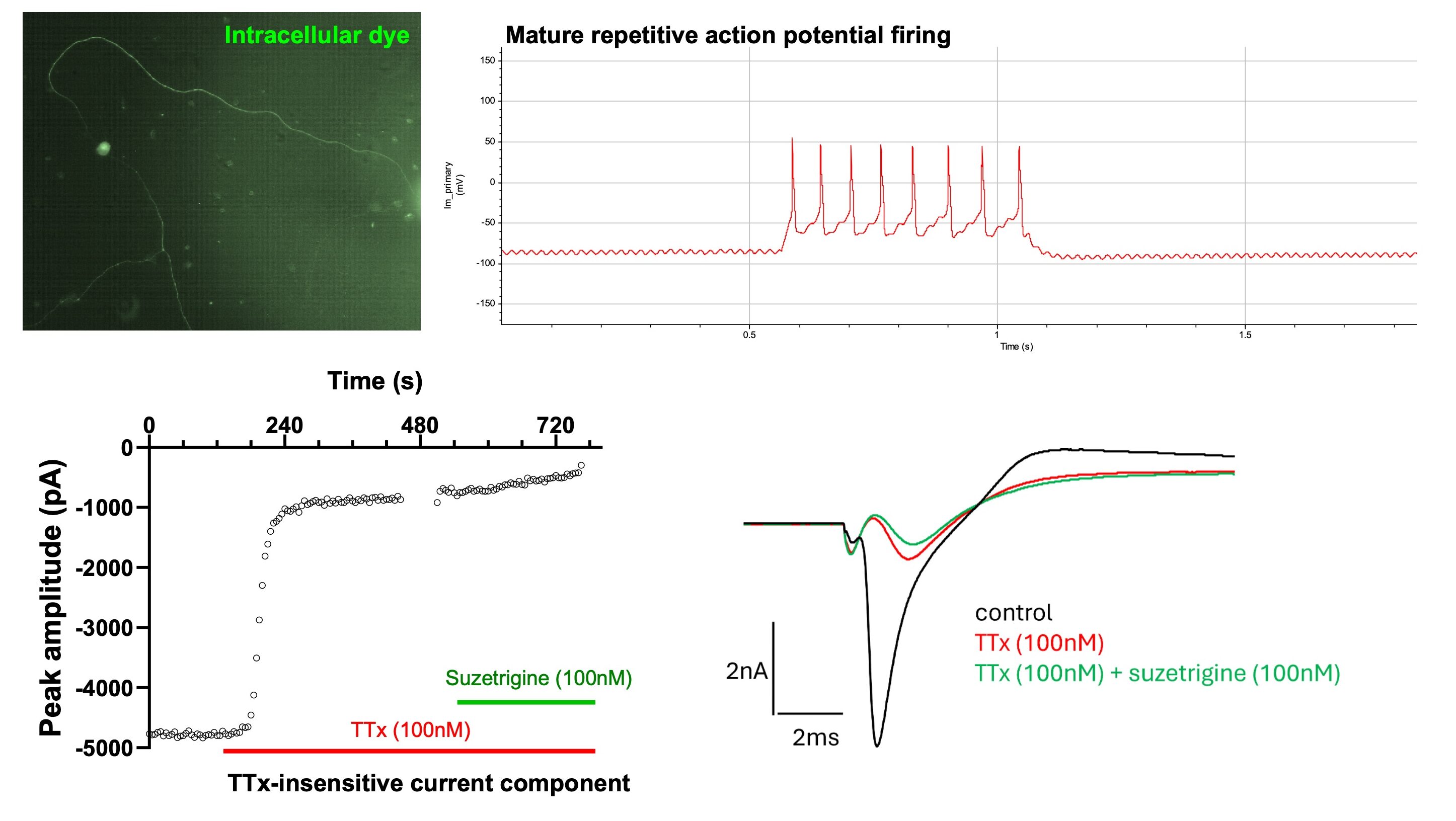
Manual patch clamp electrophysiology of iPSC sensory neurons: Intracellular dye filling shows the morphology of sensory neurons, and current injection leads to mature repetitive firing by 21 days in vitro. Example time course (and corresponding current trace, RHS) showing the partial inhibition of peak sodium current with TTx and the presence of a TTx-insensitive component.
The functional responses of these sensory neurons to various agonists were then investigated. High-throughput, single cell calcium imaging with machine-learning based automated cell body detection demonstrated calcium influx in response to nociceptive agonism of TRPV1 (capsaicin), P2X3 (α,β-methylene ATP), TRPM8 (WS-12), TRPM3 (CIM0216) and histamine receptors, but no responses were seen with DMSO control or TRPA1 agonism (cinnamaldehyde).
MEA was also used to measure local field potentials and extracellular action potentials in response to addition of the same nociceptive agonists. Similarly to the responses observed with calcium imaging, MEA showed the most action potential generation following the addition of α,β-methylene ATP, and responses to capsaicin and histamine were also observed.
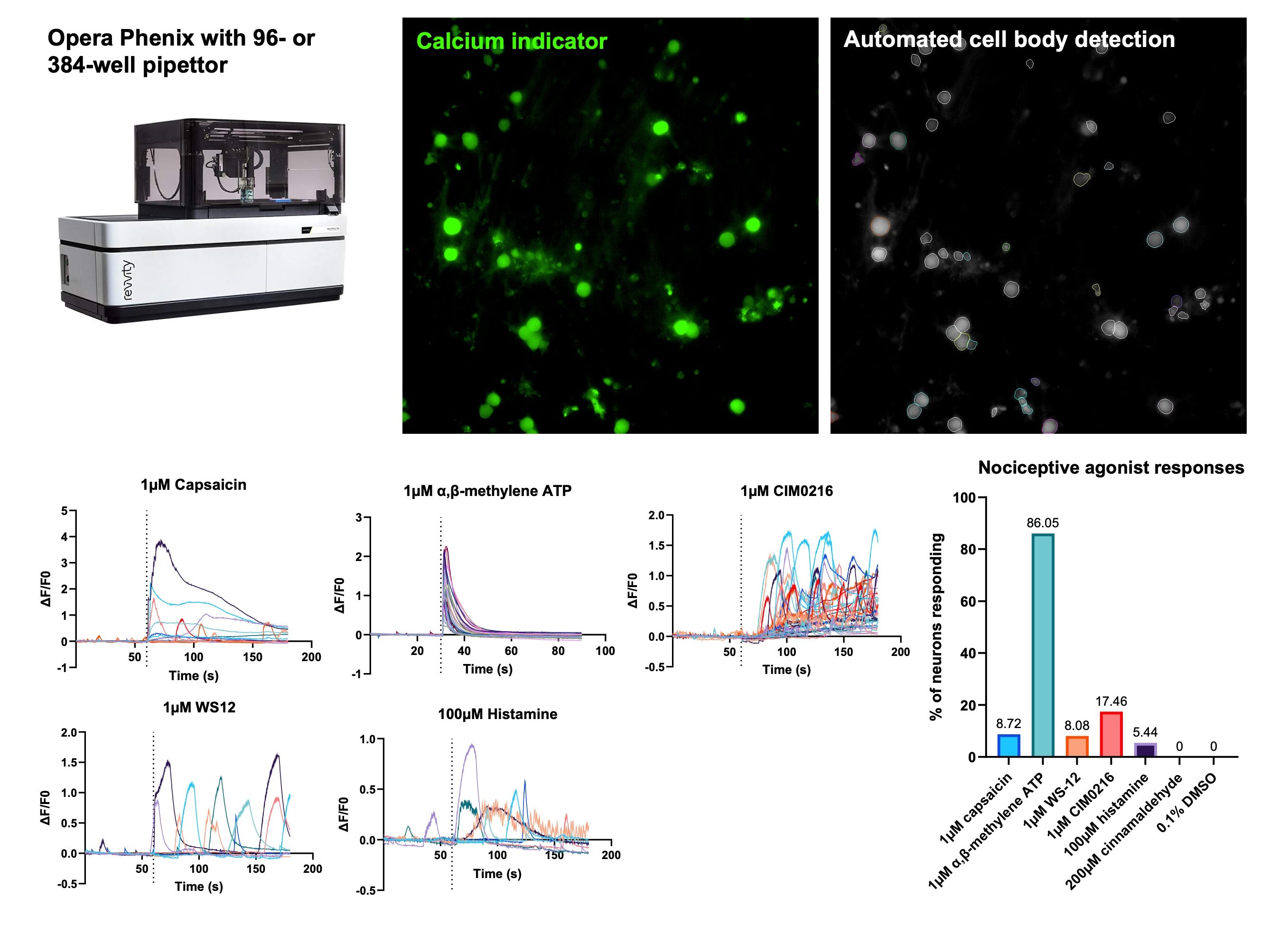
Calcium imaging demonstrates functional responses to a range of nociceptive agonists: High content microscopy and automated machine learning analysis allows single-cell calcium imaging in high throughput 96- or 384-well format to generate background subtracted calcium flux traces. Pipettor injection of various nociceptive agonists leads to ion channel activation, electrical activity and subsequent calcium influx.
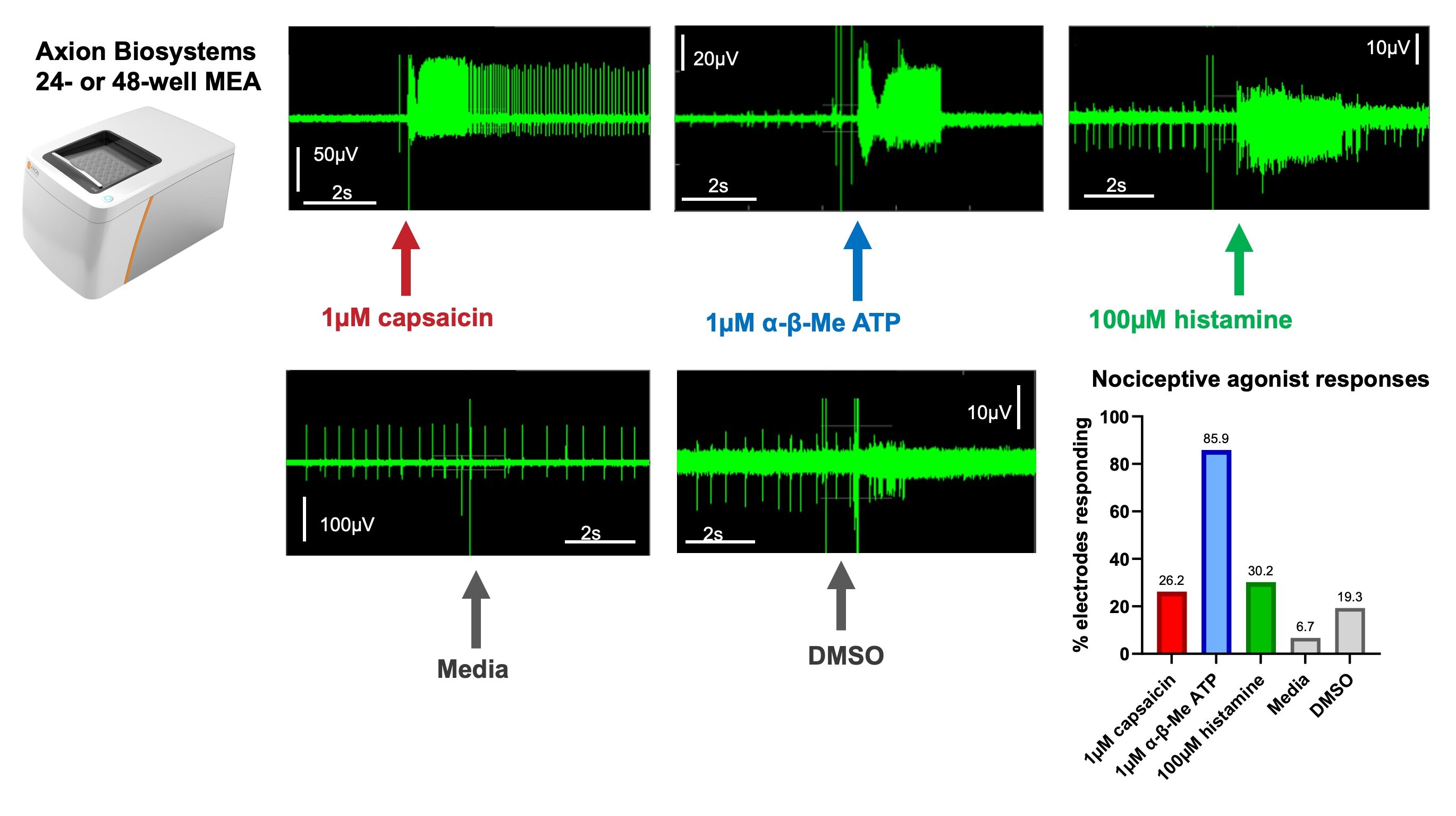
MEA demonstrates action potential firing in response to nociceptive agonist addition: MEA allows analysis of local field potentials in response to agonist addition
The P2X3 receptor is a ligand-gated ion channel that is activated by ATP when it is released from damaged or inflamed tissues, and increased expression and activity of this channel in sensory neurons has been implicated in chronic pain conditions. As over 80% of iPSC-derived sensory neurons were shown to respond to the P2X3 agonist α,β-methylene ATP, blockade of these responses with clinically developed P2X3 antagonists was carried out.
Calcium imaging was used to generate concentration response curves to α,β-methylene ATP. The mean response amplitude of all neurons in the well and the proportion of neurons responding (classed as a response amplitude greater than the mean + 10 standard deviations of each neuron’s baseline recording) both generated concentration response curves with an EC50 between 100 and 250nM.
The small molecule P2X3 antagonists eliapixant and gefapixant were then added prior to α,β-methylene ATP application. Both therapeutic agents reduced the calcium response amplitude to α,β-methylene ATP, validating calcium imaging in iPSC-derived sensory neurons as useful method for analgesic development.
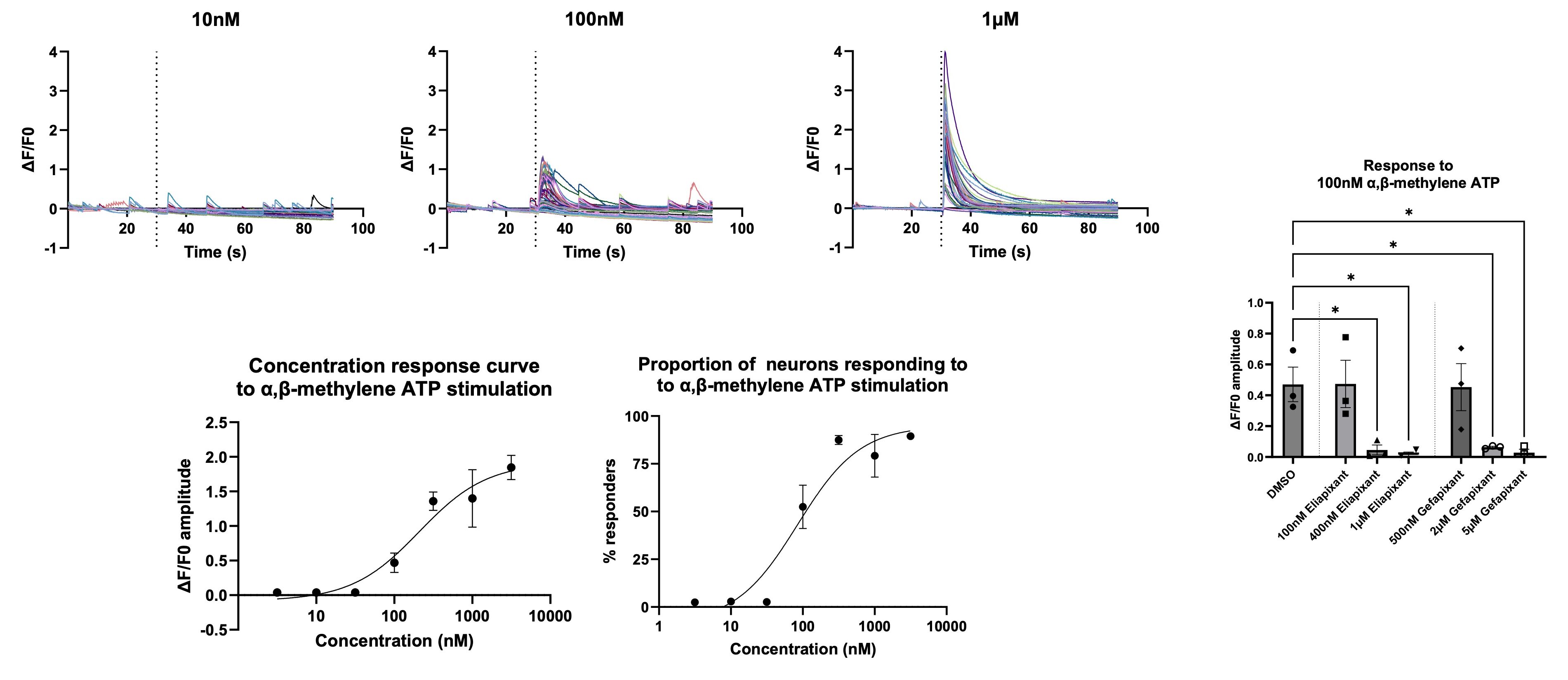
Calcium imaging of iPSC-derived sensory neurons can be utilised in drug development to investigate the modulation of evoked responses by analgesics: Sensory neurons show a dose dependent calcium flux amplitude and number of neurons responding with increasing concentrations of α,β-methylene ATP, which can be used to generate concentration response curves. These responses can be blocked by eliapixant and gefapixant, clinically relevant antagonists of the P2X3 receptor.
Increased spontaneous activity of peripheral nociceptors is a key feature of chronic peripheral pain. The effect of peripherally acting known analgesics on spontaneous activity was assessed using MEA.
The non-selective sodium channel blocker anaesthetic lidocaine, and the cannabinoids cannabidiol and tetrahydrocannabinol all significantly reduced neuronal firing frequency 1 and 1.5 hours after application. The non-steroidal anti-inflammatory drugs (NSAIDs) ibuprofen and naproxen caused a modest reduction in firing frequency. As NSAIDs act via cyclooxygenase enzyme inhibition, 1.5 hours may not have been enough to reduce levels of prostaglandins to levels low enough to see significance. Exposure to methanol or DMSO as vehicle controls caused no reduction in spontaneous firing rate. These data demonstrate that MEA recordings of spontaneous activity can successfully identify known peripherally acting analgesics, providing a valuable tool in pain drug discovery.
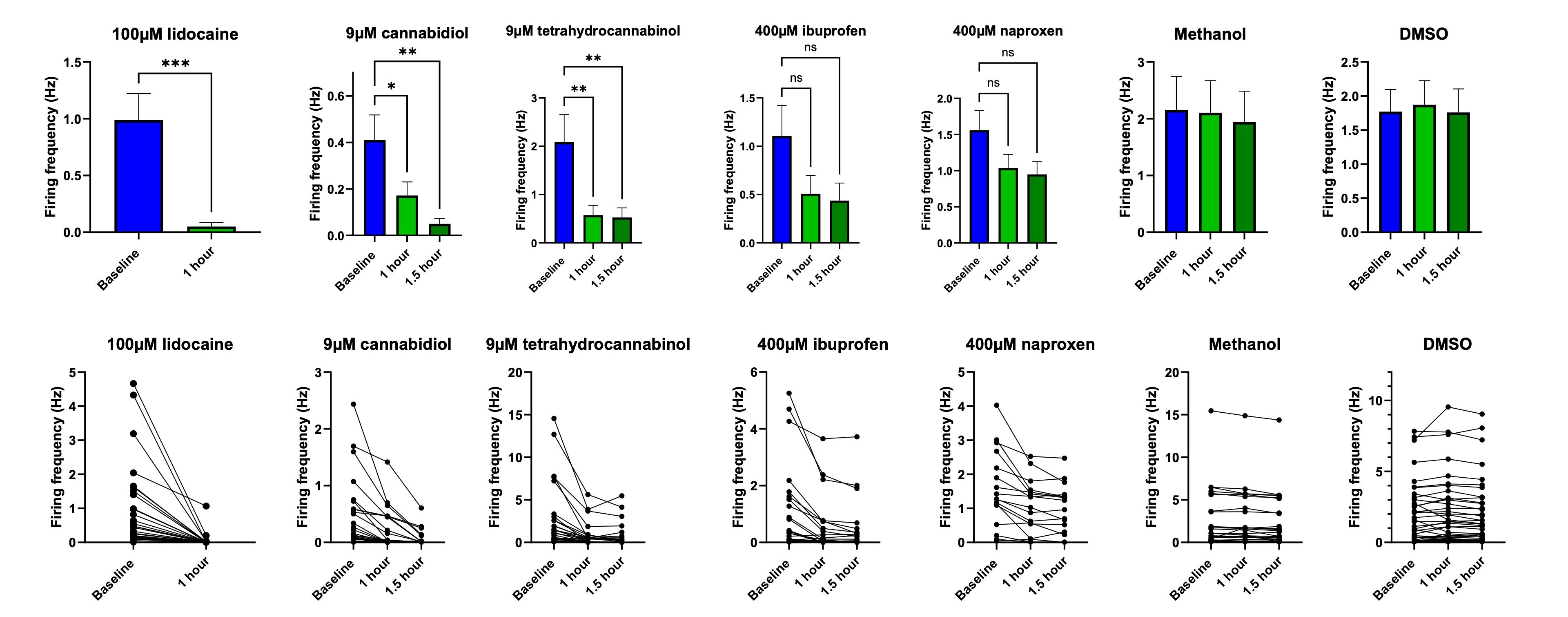
iPSC sensory neurons on MEA can be utilised in drug development to investigate the modulation of spontaneous activity by analgesics: Spontaneous firing can be assessed using MEA technology. This spontaneous firing is modulated by known analgesic agents such as sodium channel blockers, cannabinoids and NSAIDs to some extent.
A range of chemotherapeutics are known to be neurotoxic to peripheral sensory neurons, inducing peripheral neuropathic pain known as chemotherapy-induced peripheral neuropathy (CIPN). This pain is associated with cytotoxicity of the DRG neurons and degeneration of their distal peripheral axons. We observed the effect of the CIPN-causing drugs paclitaxel and oxaliplatin on sensory neurons in vitro. Neurite length following addition of paclitaxel or oxaliplatin to the media, as well as the level of cytotoxicity, were analysed over time using an Incucyte SX5. There was no cytotoxicity observed with any of the concentrations of paclitaxel used, but neurite length still decreased in a dose-dependent manner. However, oxaliplatin behaved differently, with the highest concentration inducing cytotoxicity over time that was associated with neurite degeneration, but with no effect at lower concentrations.
An AlphaLISA assay was also used to measure the concentration of Neurofilament Light (NF-L) present in the media following the imaging experiment. NF-L is an emerging biomarker that is released when nerves are damaged, and levels have been shown to correlate with the progression and severity of CIPN. Again, paclitaxel induced a dose-dependent increase in NF-L concentration, but NF-L concentration was only increased above control conditions after exposure to the highest concentration of oxaliplatin.
These findings recapitulate the neurite degeneration and NF-L release seen in patients with CIPN but also demonstrate some key differences in the mechanism of action between chemotherapeutic agents. This shows that iPSC-derived sensory neurons can model features of peripheral neuropathy in vitro and may offer insights into disease progression.
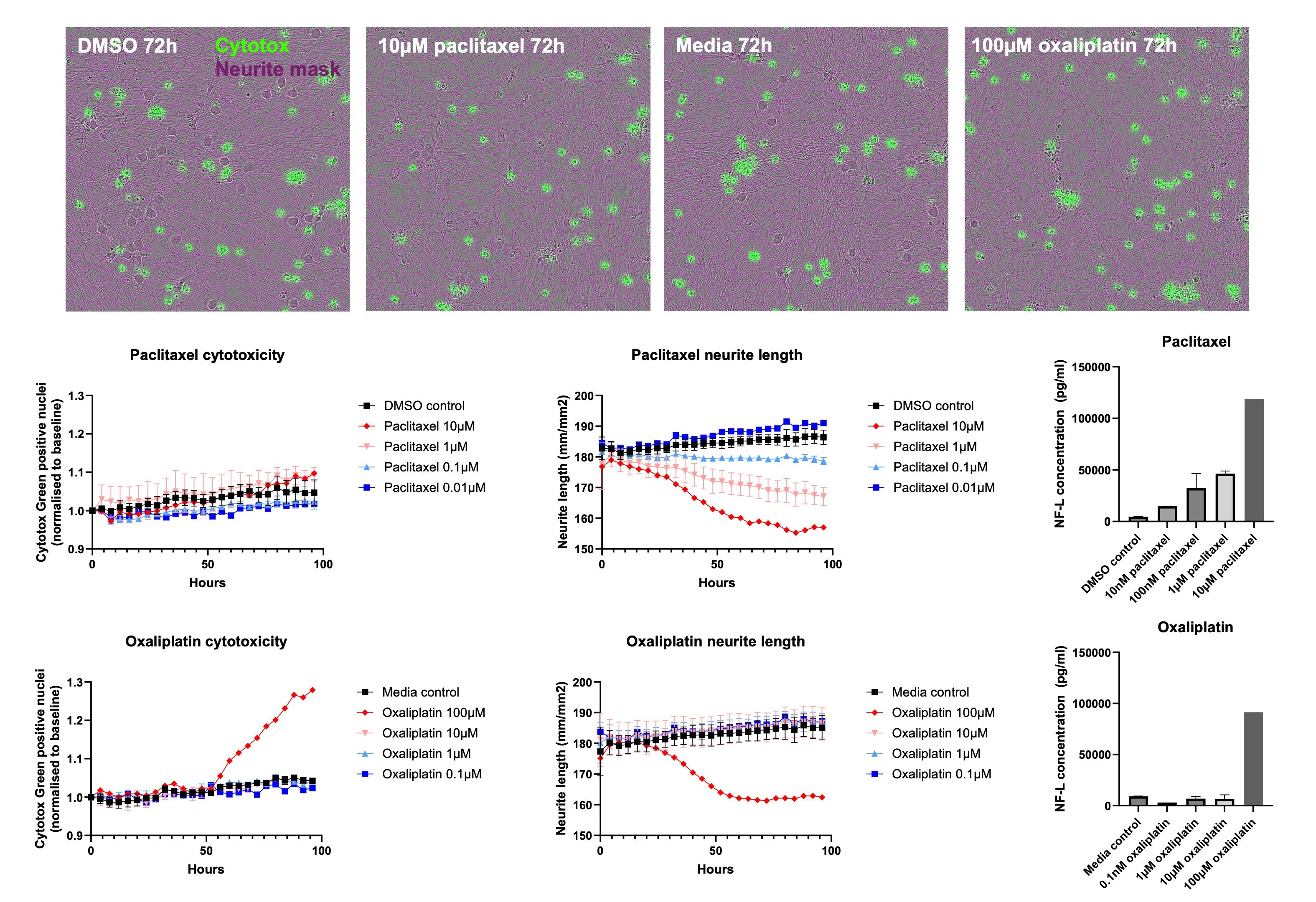
Modelling of CIPN in vitro: Time lapse imaging shows the degeneration of neurites in response to the addition of paclitaxel or oxaliplatin, though this is associated with cytotoxicity with oxaliplatin only. NF-L release correlates with the degree of neurite degeneration, modelling known features of CIPN in vivo.
We have also been able to culture sensory neurons in microfluidics devices for compartmentalised culture. Microfluidics consist of chambers connected by microchannels that are narrow enough to allow spatial and fluidic isolation of cell types but wide enough to allow neurites to project through into the connecting chamber.
We show that sensory neurons grow well in microfluidics and project neurites through microchannels into the connecting chamber. We were also able to plate iPSC-derived microglia in the connecting compartment to generate compartmentalised cocultures, as well as coculture sensory neurons and microglia directly. These complex cultures allow us to model neuroinflammation in the spinal cord which is known to maintain and amplify pain signals from the periphery. To find out more about MDC’s novel assays to monitor neuroinflammation in vitro and in vivo in the wider context of CNS disease click here.
The combined use of iPSCs and microfluidics allow more complex in vitro modelling of the nociceptive pathway. For example, the ability to model spinal cord microglial activation, the DRG to dorsal horn spinal cord connection, or the innervation of the skin and the contribution of cell types such as keratinocytes here. It also allows the application of therapeutics to only the cell body compartment or only the axonal compartment, which may better represent where a therapeutic is likely to act in the body.
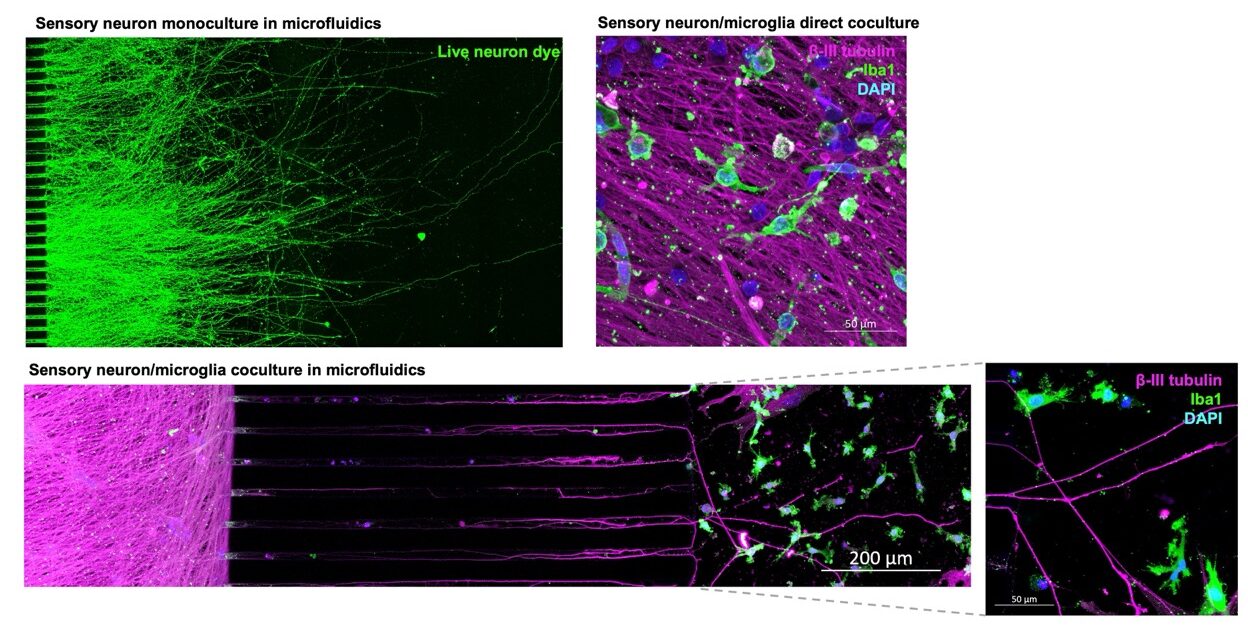
iPSC sensory neurons are amenable to complex, compartmentalised culture and co-culture with iPSC microglia in microfluidics devices: Microfluidics allows the spatial and fluidic isolation of cell types, while allowing functional connections via the axons alone. This can be applied to modelling how the DRG contact microglia in the spinal cord and how neuroinflammation here can lead to the amplification of pain signals.
This dataset developed in collaboration with MDC and ApconiX shows that iPSC-derived sensory neurons have the power to unlock translational research for novel analgesic development.
Get in touch with us to discuss your research challenges so we can accelerate your pain therapeutic research and explore how we can support your drug discovery research.
Speak to us
This is the first in a series of radiopharmaceutical blogs from Dr Juliana Maynard. Juliana is an expert imaging scientist with over 20 years’ experience in nuclear medicine and translational imaging.

In this blog, Dr Phil Auckland details how MDC has established a reproducible CRISPR-Cas9 pipeline and generated new tools to better inform nanotherapeutic design
Nanotherapeutics often fail because of Endosomal entrapment. In this blog by Dr Phil Auckland, we explore how MDC are using real-time imaging to reveal why.