The Multiple Sclerosis (MS) Society is the leading UK charity for people affected by MS. Their number one organisational goal is for everyone with MS to have access to effective treatments to slow, stop or reverse the accumulation of disability. In support of this goal the MS Society aims to accelerate the translation of medicines into the clinic.
To enable new treatments to reach patients faster, the MS Society and its expert consortium wanted to investigate drugs used in the treatment of other diseases as potential clinical candidates for repurposing in MS.
Leveraging existing scientific knowledge and insights in MS, the MS Society and the expert consortium followed an expert-led approach to compile and then prioritise a list of candidate drugs for a clinical study in MS, taking into account drug’s characteristics such as efficacy, safety and toxicology data.

To complement their approach and validate their findings, the MS Society needed additional expertise to ensure that any potential additional drug candidates were not overlooked. They required a mechanism to systematically identify drugs that could potentially slow or stop the progression of MS whilst collecting relevant data needed to enable their prioritisation for the clinic. This would also serve as an independent parallel validation of their identified candidates, and would have been a time consuming and resource-heavy exercise if conducted manually.
Medicines Discovery Catapult (MDC) worked closely with the MS Society to develop a data-driven approach to the identification of potential drug candidates for MS using modern machine learning approaches.
This involved:
The resulting list of drugs with potential application to MS was then shared with the MS Society and compared to the list generated manually by the expert consortium. The lists overlapped well, and the machine-learning approach identified approximately 300 further candidate drugs for investigation by the MS Society.
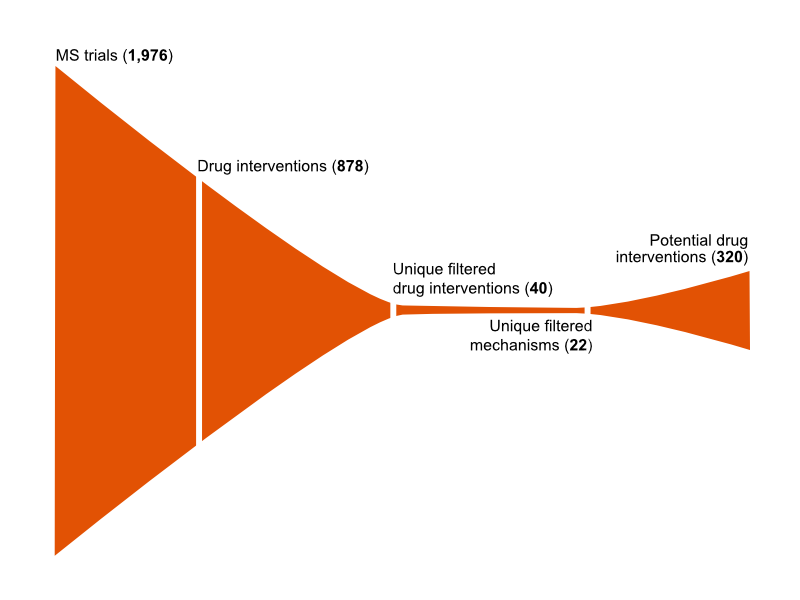
The MDC informatics workflow identified 878 drugs tested in 1,976 MS trials and was able to filter these by distinct drug intervention and mechanistic classes in order to prioritise the top 320 drug candidates with potential to stop or slow MS progression
To enable the prioritisation of drugs within this new list of 300 proposed drugs, MDC retrieved extensive biological data associated with each candidate drug. This included safety and toxicology data, patent information, cost, side effects, contraindications, the ability to cross the blood-brain barrier, potential drug interactions with standard MS treatments and the available administration routes for each drug.
To then facilitate the direct comparison of drugs using this data, an interactive web page interface was designed and supplied to the MS society. This would enable the MS Society expert consortium to easily rank drug candidates by weighting the different factors represented in the metadata.

The interactive web page interface designed and supplied to the MS Society
Through the independent, data-driven analysis conducted at MDC, the MS Society’s expert consortium was able to validate their list of potential drug candidates. This provided increased confidence in their data and allowed the identification of further potential clinical candidate drugs. The additional data gathered for each candidate drug also provided the expert consortium with additional information to support future prioritisation of drug candidates and recommendations for drugs to enter the MS Society’s clinical trial platform.
To further support the expert consortium, the MS Society and MDC are arranging a workshop to take place in November 2019. The MDC informatics team members will support the expert consortiums interrogation of the data produced during this project.
Ultimately, this project has supported a strategic drug prioritisation exercise. It has provided further confidence that the drug candidates selected to enter the MS Society’s clinical trials platform have the best chance of success and the potential to provide patients with better treatments faster.
“It has been a pleasure working with MDC on this project, which has given the MS Society an opportunity to utilise the impressive Informatics skills and capabilities at MDC to complement our own academic-led work. Our treatment selection initiative is focussed on achieving impact for people affected by MS through accelerating the development of treatments, and MDC have been an effective, responsive and collaborative partner in helping us make progress.”
Dr Sorrell Bickley, Head of Biomedical Research, MS Society

MDC Helping UK SMEs access tissue samples, a collaboration with Tissue Solutions.