
Medicines Discovery Catapult and the BioIndustry Association stated in their 2018 State of the Discovery Nation Report, that:
“… over 80% of SMEs surveyed agreed that access to biosamples is hugely important for commercial development” and “… as many as 80% found accessing UK samples unexpectedly difficult…”
A major recommendation of this report was that actions need to be taken to improve sample and data access for all translational scientists. Hopefully, the information provided in this article will provide some assistance in this respect.
Most biosamples originate in the public sector in hospitals, clinics and collection centres. They are stored in biobanks, which may be called academic biobanks, hospital-based biobanks, or population biobanks.
For researchers in the private sector, there are two main ways of accessing human biosamples for research. Firstly, there is the direct route, which involves obtaining samples from biobanks. Secondly, there is the indirect route, which involves paying a commercial intermediary organisation to source the biosamples for you. These two routes are shown in this diagram:
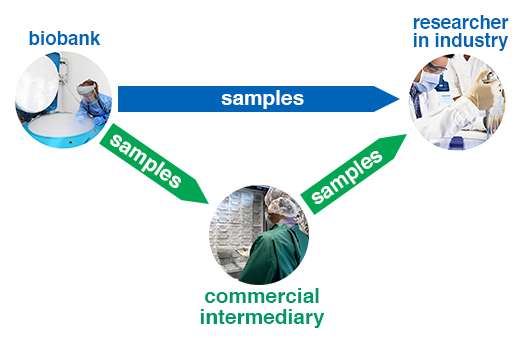
Diagram showing direct and indirect routes for access to biobank samples
To take the direct route, you need to identify a public sector biobank that is willing to supply the kind of samples you need.
A good place to start looking is the UKCRC Tissue Directory – an online directory, which allows you to search for biobanks with the samples you need, or biobanks that can start a collection for you. If your search is not successful, you may want to try the BBMRI-ERIC Directory, a similar but larger directory. These directories will provide the contact details you need to explore further and decide whether the biobank you have found is a good fit.
Whether you find a suitable biobank by this route will depend very much on what samples you are trying to source. If you are searching for a common disease type, with simple clinical information, you may find a biobank very easily. On the other hand, if you are searching for a rare disease, or you need extensive clinical information, your search may take much longer.
If you need samples to be collected in a very specific way, you may need to find a biobank that can start a prospective collection for you. In this case, there is an additional time delay while you wait for your collection to be completed.
Another important factor is whether the biobank’s access policy will allow industry access and, if they do, what are the conditions. In some cases, biobanks will only allow access to the private sector if they are involved in a research collaboration with the institution to which the biobank belongs. This information is frequently not advertised publicly, as reported recently by Langhof et al (2017). So, before investing any time in an application, it is best to contact the biobank to ensure that access will be possible on acceptable terms.
You also need to bear in mind that, there will be a cost recovery fee for samples. This covers the cost of all the technical and administrative work needed to collect, process, store and provide the biosamples and associated data.
Commercial intermediaries will generally be either:
The commercial intermediary needs to charge for the sourcing service it provides. Sometimes this means that commercial intermediaries are more expensive. However if, for example, samples have been sourced from overseas, they may come from countries where salaries and cost of living are lower. In such cases the cost recovery fee for sample processing will be reduced and hence the overall fee may also be reduced.
Whether you should choose the direct route or the indirect route to source your biosamples will depend on a number of different factors including:
Hopefully, by weighing up the advantages and disadvantages described in this guide, you will find the access route that works best to meet your company’s biosample needs.

Dr Robert Hewitt is a Biobanking Specialist.
Previously, Robert worked in cancer research, both as a lecturer in pathology, at the University of Nottingham; and as a visiting fellow, at the National Institutes of Health. Since 1998, he has established/managed biobanks in London, Saudi Arabia, Singapore and Luxembourg. He was President of ISBER in 2007 and moved on to co-found ESBB in 2010, serving as the sole employee until 2017. After one year of industry experience, working for Trans-Hit Biomarkers, Robert now works independently as a consultant, promoting cooperation between academic biobanks and industry. He is active on LinkedIn and runs the ‘Drug Targets & Biomarkers’ group.
Preclinical to clinical, building a translational link between in vitro and in vivo models of neuroinflammation for drug discovery.