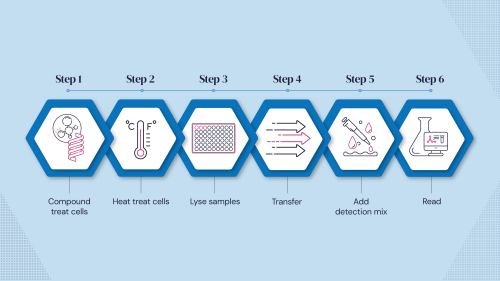
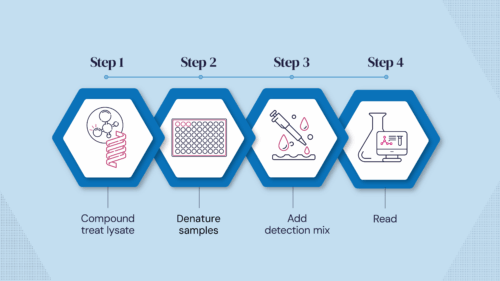
We have developed a proprietary cell lysate‐based chemical protein denaturation technology to confirm protein target engagement. This is accomplished by assessing binding-induced stabilisation in a simple mix-and-read, scalable, cost‐effective assay.
Determining ligand target engagement is an essential process for hit discovery and lead profiling, where simple and rapid approaches are highly valuable tools in the drug discovery toolbox. CPSA is unique in that it only requires mix-and-read steps within a single assay plate, giving a streamlined, cost‐effective workflow without transfer steps. The use of cell lysates avoids potential difficulties with the purification of protein targets.
CPSA is adept at handling diverse needs, from rapid evaluation of a few lead compounds to 1536-well screening of large-scale libraries. As it does not rely on target activity, CPSA has the potential to identify novel series that bind outside of the active site and move into novel chemical space.