The Advantages of Tumour DNA Detection in Liquid Biopsies
Circulating tumour DNA (ctDNA) analysis in liquid biopsies addresses an array of questions pivotal for cancer research and treatment.
Benefits of this technique include:
- Detection of tumour burden
- Measurement of treatment response
- Prediction of therapy outcomes
- Patient stratification for optimised treatment
- Early identification of minimal residual disease ahead of imaging techniques
All these insights are derived from simply a few millilitres of blood, making liquid biopsies quicker, less expensive, and safer than conventional biopsies. Most impressively, they capture DNA shed from the entire body, offering a broad and in-depth perspective. Along with its low risk and cost, high data yield, and comprehensive coverage, this tool is ideal for longitudinal monitoring of patient response, remission, or relapse.
Depending on the research question, ctDNA can be analysed in two ways:
| Global (by sequencing) | Targeted |
|---|---|
| This involves sequencing the entire genome, exome or selected oncogenes to identify cancer-associated mutations.
Pro: Unbiased analysis |
Here, specific mutations are targeted. Digital PCR, for example, has proven highly sensitive in identifying allele frequencies of 0.1% or lower.
Pro: High sensitivity |
Optimised Analysis of ctDNA
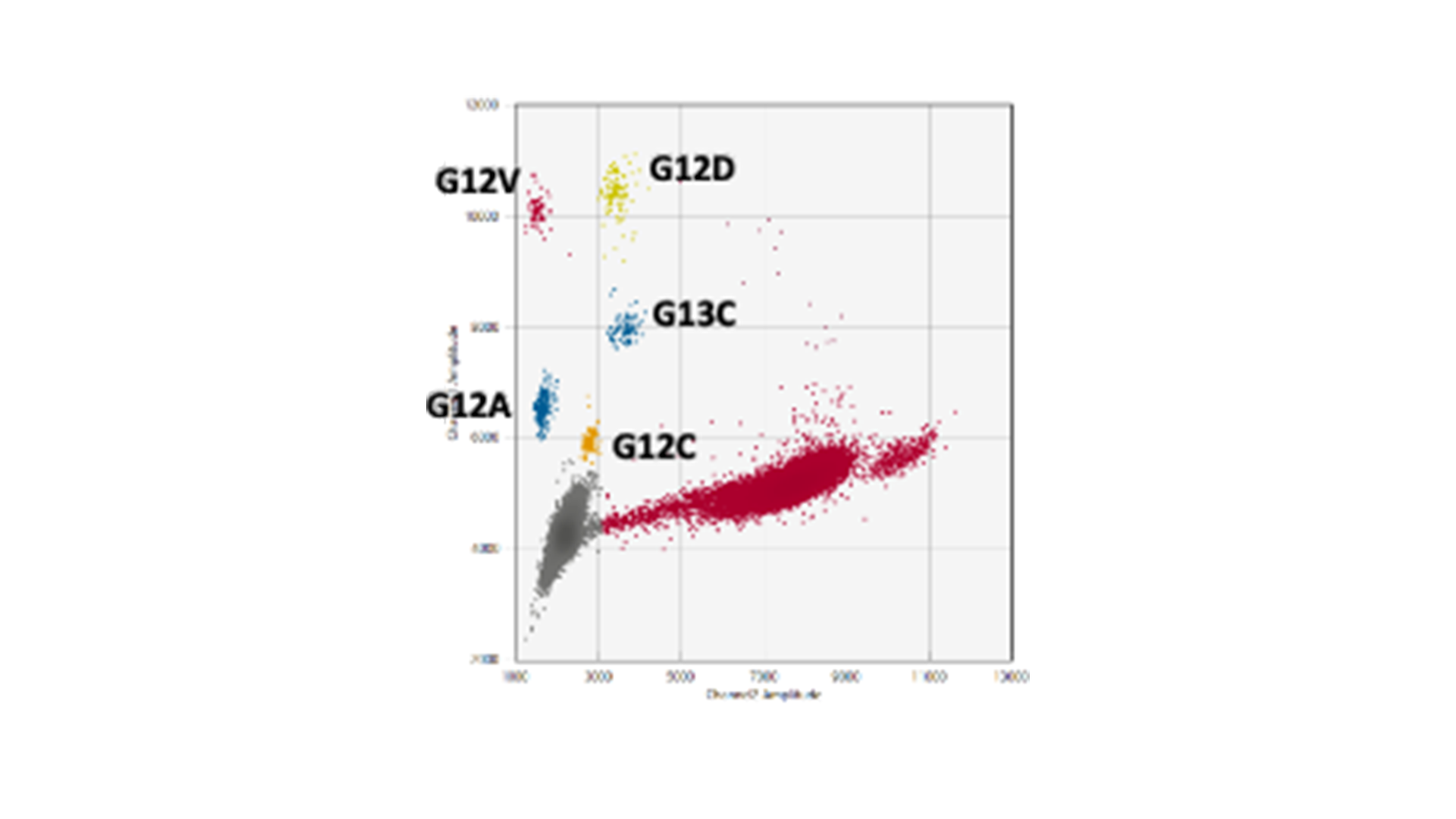
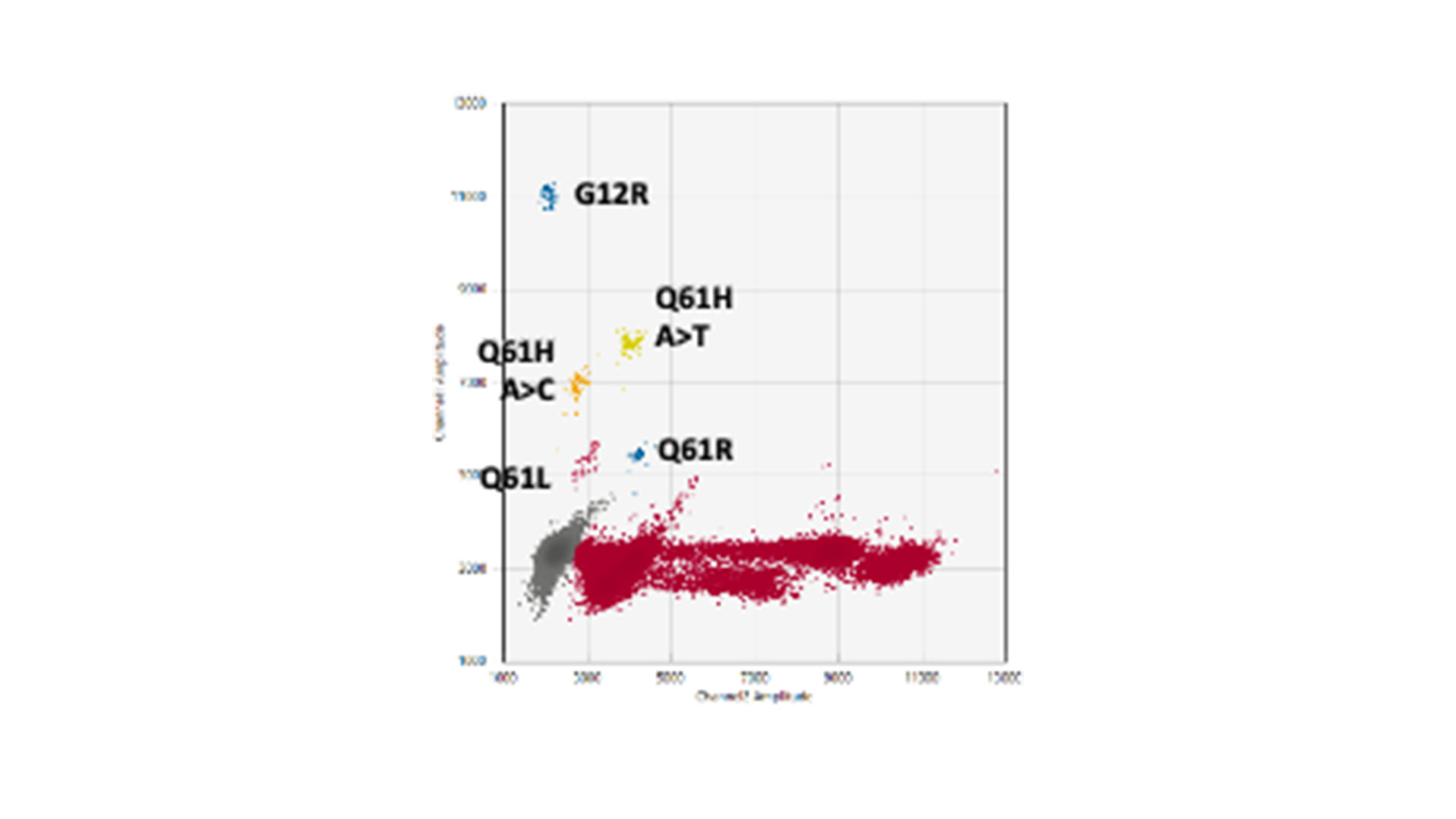
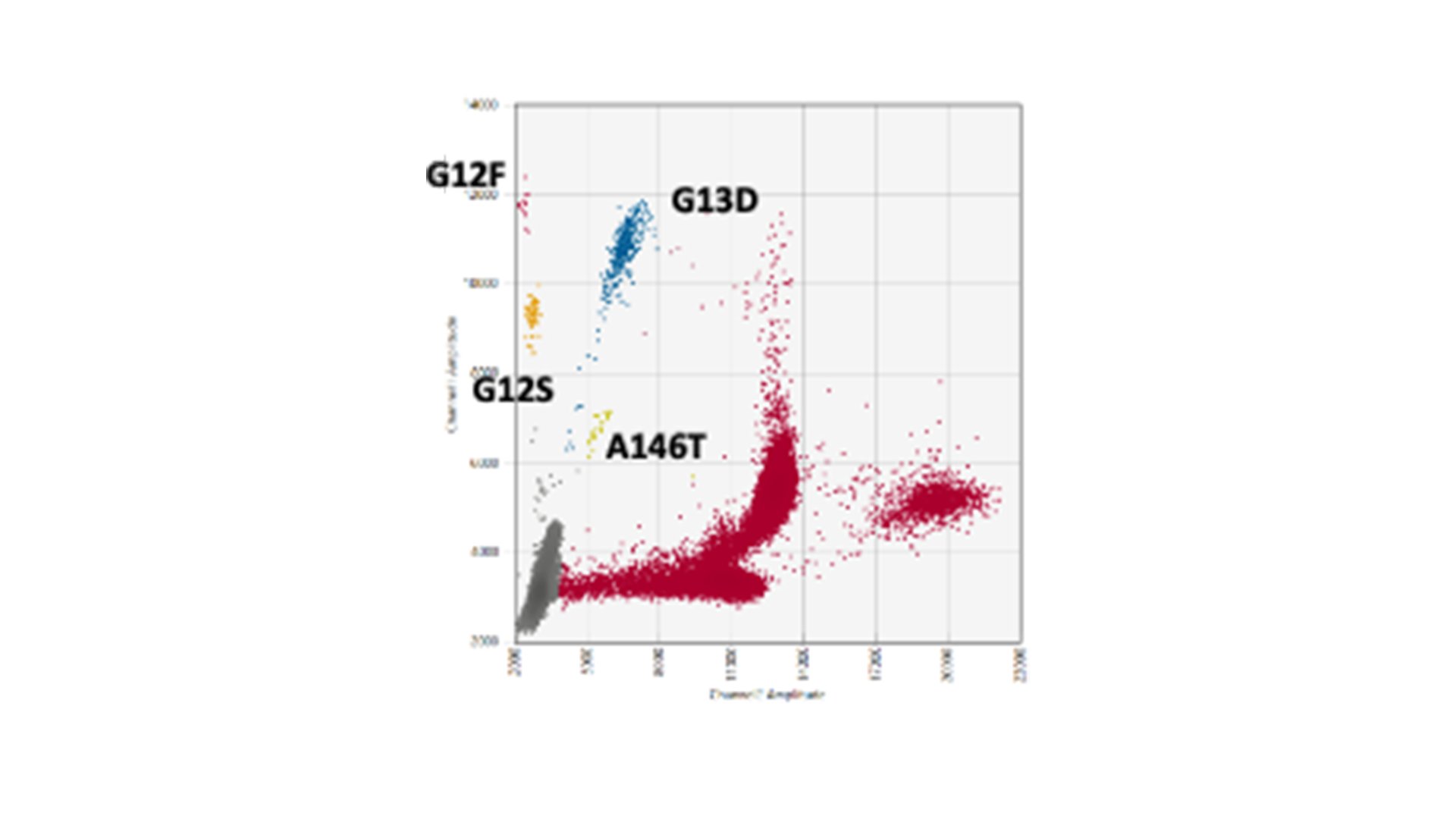
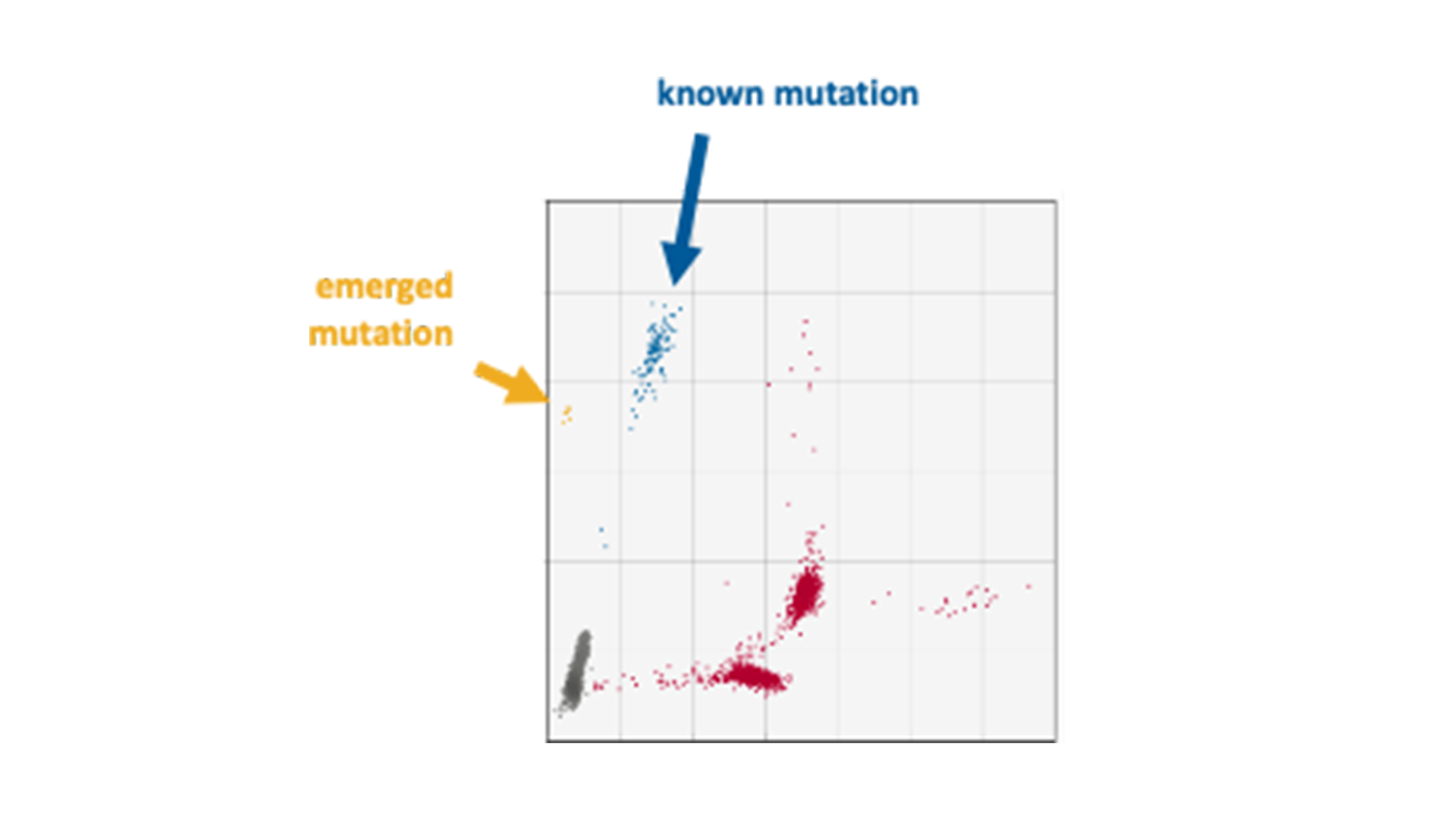
The genes KRAS and BRAF, which control growth and division of normal cells, are at the epicentre of genetic alterations in cancer, with widespread mutations turning these genes into serious disease accelerators. BRAF often fuels the disease with one notorious mutation, scientifically designated as V600E, while KRAS has a more kaleidoscopic spectrum of genetic transformations that render it into an active oncogene. Striding forward in our advanced research, MDC successfully replicated and validated multiplex droplet digital PCR assays. These assays zero in on the formidable BRAF V600E mutation and fourteen high-frequency KRAS mutations.
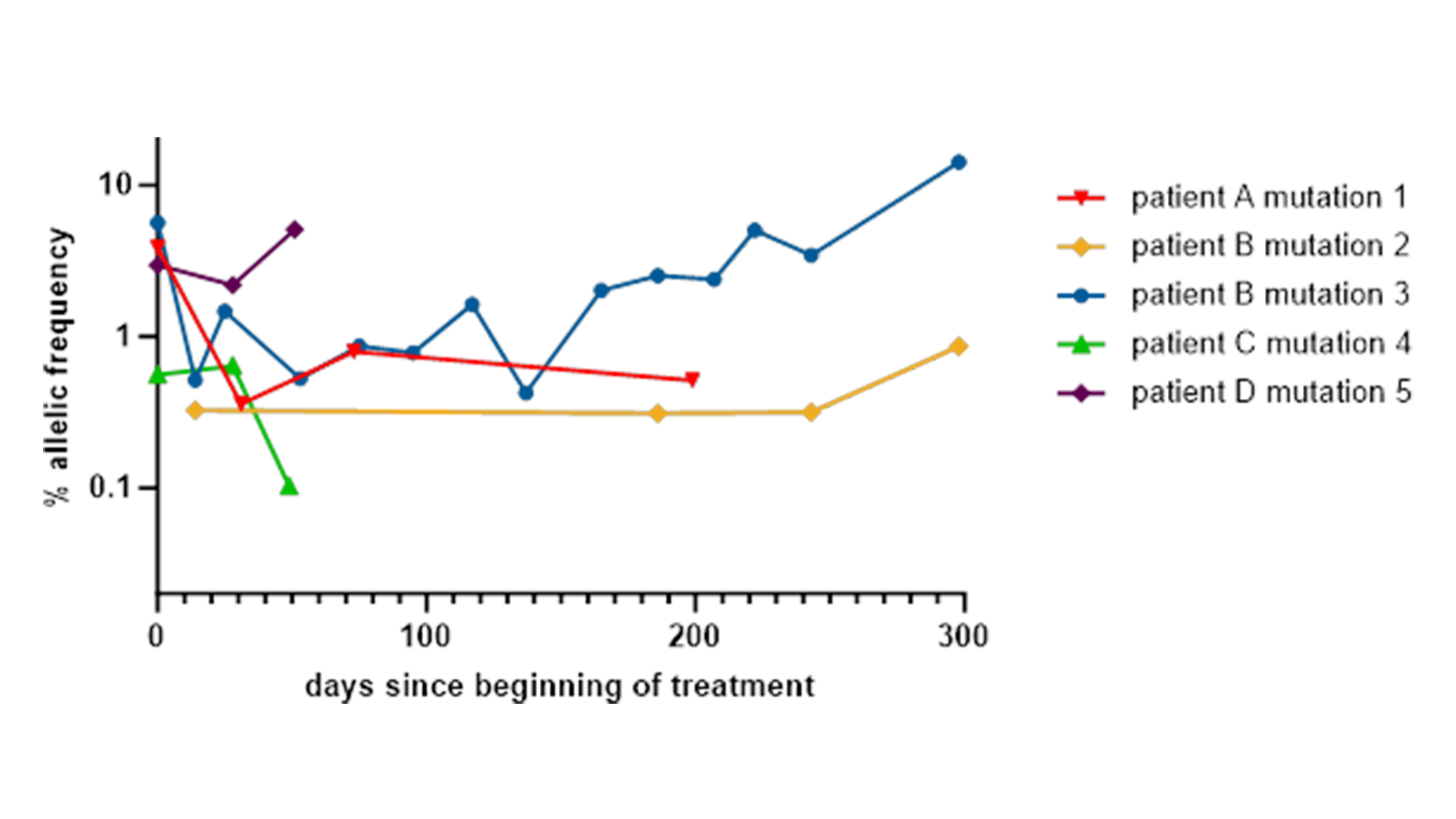
Our team of experts recently used these multiplex assays in a project with an MDC partner that involved the analysis of longitudinal samples from a phase I drug trial. Genetic mutations were found in the diagnostic samples and even identified a new KRAS mutation
Our multiplex assays facilitate the study of fourteen KRAS mutations using merely three reactions rather than one for every mutation. This eliminates the dilemma of how to best utilise the limited ctDNA sample.
Customised and Differentiated Personalised Medicine Development Solutions
You might be thinking, “Well, this isn’t ground-breaking – I’ve seen KRAS G12/G13 screening kits out there doing a similar job.” True, these kits do test for the most prevent mutations all in one go. However, while they can confirm that your sample contains a mutation, they focus on only the top few mutations, and they fall short at pinpointing exactly which mutation that is. This distinction could hold the key in discerning cancer’s genetic makeup and, consequently, its strongest or weakest point.
At MDC, we provide tailored and differentiated tests rather than generic services. Whether you’re focusing on BRAF and KRAS driver mutations or any specific set of gene mutations, our extensive experience in digital PCR assay design and validation enables us to develop bespoke digital assays to address your research needs.
Multiple companion diagnostic tests are already green-lighted for pinpointing cancer-driving mutations in ctDNA. They play a crucial role in identifying the cohort of patients with the highest odds of responding to a particular treatment. This strategic approach optimises resource allocation, saving substantial time and money. But beyond material resources, crucially it spares patients from the physical tolls of ineffective therapies. So, it’s not just about enhancing healthcare efficiency, it’s about reinforcing patient-centric care where wellness and well-being take centre stage.
By leveraging this advanced technology with MDC support, you can strategise patient stratification, treatment and monitoring more effectively in your drug development efforts.