Why is biomarker identification so important? Clinical trials are expensive with each phase becoming more expensive as the number of subjects increases, and the attrition rate is high due to a lack of clinical efficacy. Data from the AZ pipeline have shown that programmes that have a Pharmacodynamic (PD) biomarker associated with them, where target engagement or proof of mechanism has been demonstrated, have a better chance of success and proceeding to phase 3 clinical trials or launch.
PD, or Response, biomarkers included early in the preclinical stages of the drug discovery process can be used to confirm target engagement. Informative biomarker assays can be translated to the clinical phases where they can add value and provide proof of mechanism and help inform the sponsor and support their go/no go decisions.
Modulation of the immune system unpins treatments across a range of therapeutic areas, from infectious disease to autoimmunity and immuno-oncology. There are a number of powerful techniques available to measure biomarkers of immune function. For a given study, the experimental solution needs to be selected based on an understanding of the immune component which is being targeted and the logistics of the particular trial.
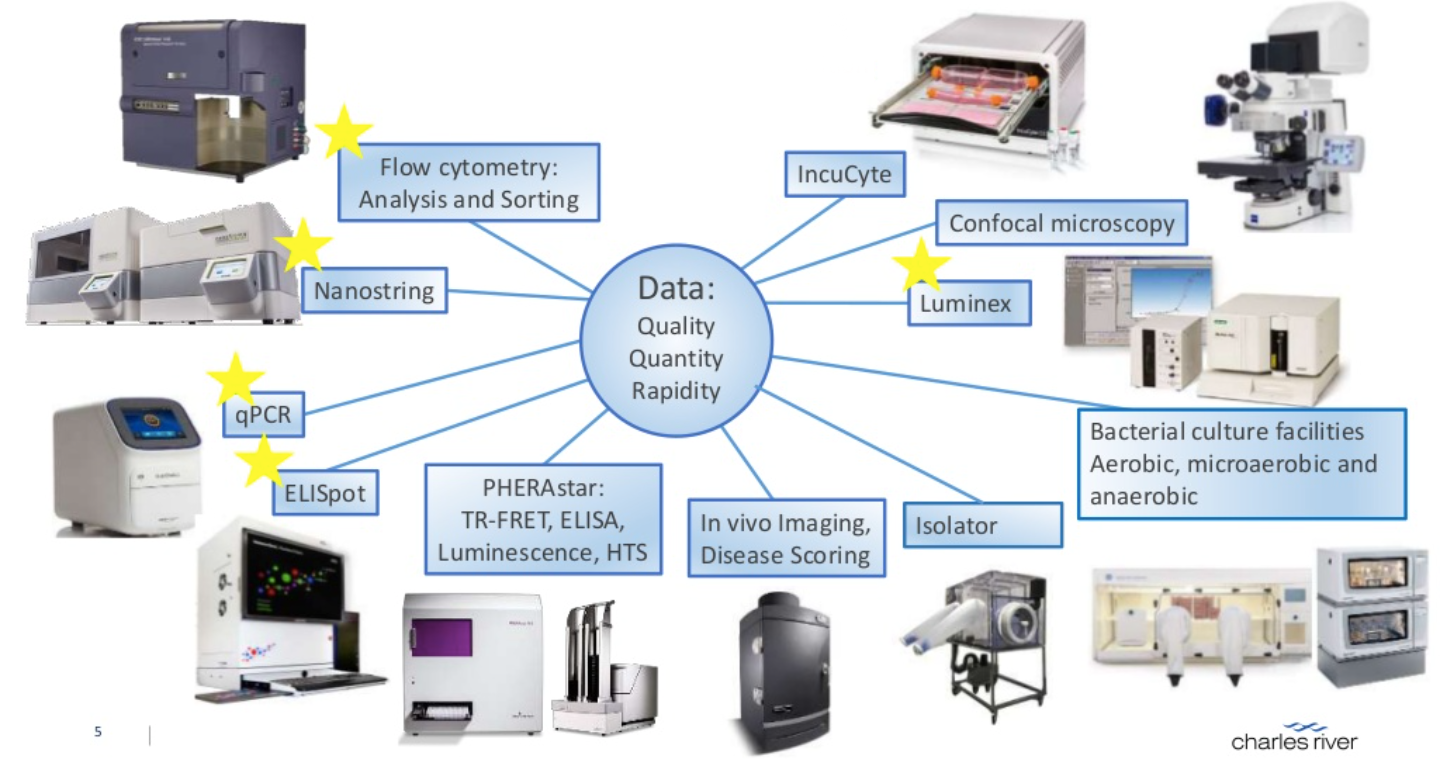
In the context of PD biomarkers, the aim is to establish a ’window of effect’ between the biomarker level following modulation by the test compound of interest. A range of experimental platforms can be employed to identify immune biomarkers. For example, ELISA or multiplex platforms (e.g. Luminex) or can be used to identify protein products stimulated by an immune response, such as an antigen-specific antibody titres or a cytokines/chemokines produced in response to an antigen. qPCR or nanostring can be used to study the molecular ‘message’ (mRNA or DNA) for a particular biomarker in a biological sample or to assess the modulation by sample treatment or stimulation. Nanostring technology is a powerful technique that can measure the expression of up to 800 genes simultaneously, profile them and the results used to select a smaller panel of appropriate biomarkers, which can be validated by higher throughput methods. At the cellular level, ELISpot can be used to confirm the success of increasing the frequency of antigen-specific T cells in vaccination studies, whilst flow cytometry is a powerful technique that can be quantify the frequency of multiple subsets of immune cells simultaneously in a biological sample. The technique selected for biomarker identification, validation and quantification will be the most appropriate for the needs of the study.
Sample logistics considerations are also essential for successful biomarker identification – how will be samples be taken, how will they be stored, how will they be shipped? This is particularly important for a pre-clinical assay to be successfully translated for use in a clinical trial. A clinical assay will typically be preceded by a validation phase which will mimic the conditions which the trial samples will be exposed to – such as exposure to freezing or fixation – to stabilise the markers before testing. Finally, the assays require fit-for-purpose validation, including confirmation of parameters such as how the standard curve performs and the intra- and inter- assay precision, which will allow batch analysis of samples to be done.
Immune biomarkers can be integrated into the drug discovery process to confirm target engagement (or proof of mechanism) from pre-clinical through to clinical phases. An optimal strategy requires informed choices regarding what immune parameter to measure, how to measure it and how much validation is necessary. Ultimately, PD biomarkers can impact positively on project success rates.
This article is based on Russell’s talk from the MDC Connects webinar series. Watch the session Russell took part in – Developing a Biomarker Strategy
About the author
Russell Garland is the Group Leader for Analytical Services at Charles River Laboratories. He is a member of the British Society of Immunology and has 20 years of experience working with immunological assays. Russell was awarded his PhD in 2000 by the University of Bristol. The activities of Russell’s group spans readouts of immune modulation in both pre-clinical and early phase clinical phases of drug discovery programmes. He is based at CRL’s Portishead site in the UK, which specialises in immunology and infection. Russell is Analytical Project Manager for their clinical services, with a particular focus on pharmacodynamic (response) biomarkers.