Principles and modelling of pharmacokinetic and pharmacodynamic relationships
Mathematical modelling is currently hard to avoid due to headlines regarding COVID-19. Models are being used to predict situations such as the worlds response to COVID-19, how many will die globally from the pandemic, etc., and not all the headlines are complimentary. At the start of COVID-19, these models were simple models based mainly on assumptions with little data. As more data become available, the assumptions can be replaced by data and the models become more robust, however this incurs both time and financial costs.
XenoGesis aims to offer simple, but useful modelling of pharmacokinetic (PK), pharmacodynamic (PD) and integrated pharmacokinetic-pharmacodynamic (PK-PD) modelling requiring minimal data and therefore cost. This can allow for efficient design of pre-clinical PK-PD studies and, through the integration of predicted human PK, predictions of efficacious dose regimes in the clinic.
How does XenoGesis do this?
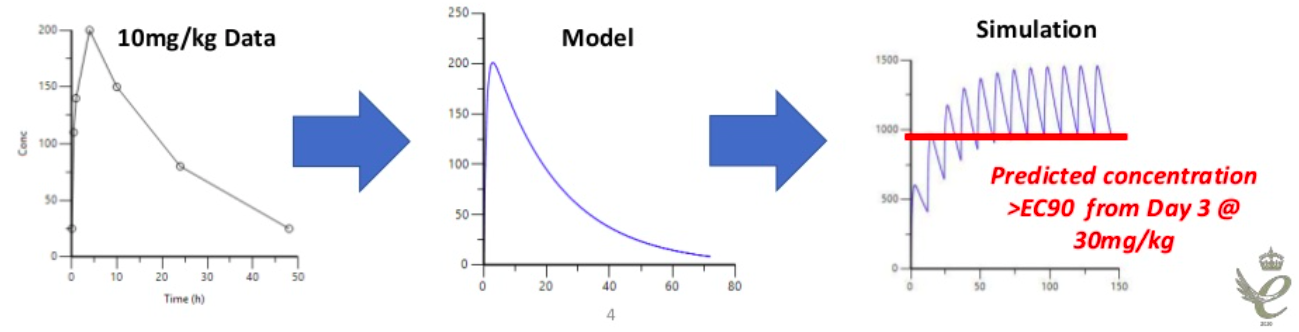
PK modelling can be useful in early stage drug discovery. If the plasma exposure is known from a single 10 mg/kg dose in a mouse, modelling could be used to predict what could happen to trough concentrations with a twice daily 30 mg/kg dose, which could then help define a hypothesis for the pharmacology stage.
PK, PD and PK-PD – what’s the difference?
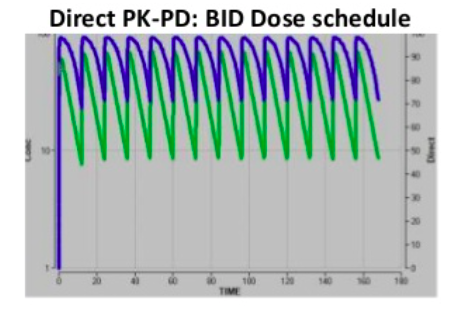
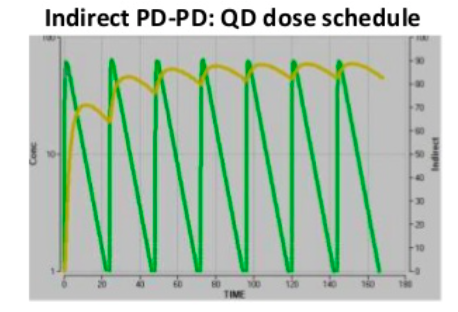
PK measurements (concentration vs time) can be often be described using a single compartment IV model with 2 parameters: clearance, and volume. These are then plotted with observed data and a linear line is drawn. When the data do not fit this linear line, and show over and/or under prediction, a more complex model is required with additional compartments e.g. volume (V2) and clearance. In addition, an oral compartment can be included adding further complexity to the model. To this a PD effect can be added (effect versus concentration) and a direct PK-PD relationship can be observed i.e. the integrated relationship between the plasma exposure time (PK) and effect versus concentration (PD), for a given dose, and route of administration.
Direct PK-PD models are the simplest, meaning at all time points the concentration in plasma is directly related to the effect. Indirect PK-PD occurs when there is a delayed response in vivo and the maximum effect occurs later. The plots show the same underlying PK and EC50. The direct PK-PD needs twice daily dosing for a >70% effect, whereas an indirect PK-PD achieves >80% effect from once daily dosing. A hysteresis loop indicates 2 different response levels for one drug concentration.
XenoGesis believe studies should be designed to integrate all available knowledge to test a hypothesis using dose levels and samples that investigate concentration effect whilst also investigating time dependence allowing indirect PK-PD to be seen. Models integrate knowledge of drugs and help support decision making throughout the life time of the project increasing in complexity and accuracy with time.
To find out more about PK-PD, the DMDG run a 2-3 day residential PK-PD course (info@dmdg.org).
This article is based on Graham’s talk from the MDC Connects webinar series. Watch the session Graham took part in – Understanding the PK / PD Relationship:

About the author
Graham Trevitt is CSO and heads up the scientific team at XenoGesis. He joined XenoGesis in 2015, bringing over 14 years’ industrial drug discovery experience, as well as a track record of delivering pre-clinical drug candidates in oncology and inflammation through integration of DMPK properties into compound design to increase the probability of creating successful drugs. Prior to joining XenoGesis, Graham worked at UCB for 8 years before joining Almac Discovery in 2009. Graham graduated from the University of Nottingham with a PhD in Synthetic Organic Chemistry and went on to complete his Postdoctoral Research at the University of Geneva.