Evaluation of clinical and pre-clinical pharmacodynamic endpoints
Preclinical and clinical imaging endpoints can be used for key decision making throughout drug development through to regulatory approval and are essential to the drug development process.
Pharmacodynamics and imaging
In pharmacodynamics, imaging offers a non-invasive opportunity to visualise and quantify clinical response to a drug. In oncology trials, PD endpoints can be used as surrogate biomarkers of a clinical response.
There are a variety of different imaging modalities in preclinical and clinical use, as shown below. Each modality has different ranges, spatial resolution, and depth of penetration, alongside advantages and disadvantages, for example, the need for complex technology or bolus contrast.
Case study of preclinical imaging PD using FDG-PET
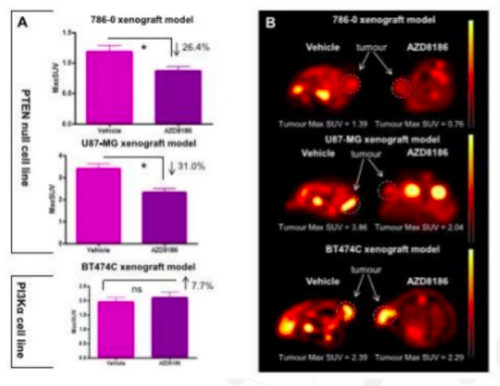
Hypothesis to test: Tumours with a PTEN deficiency will benefit from the novel oncology drug (AZD8186) and 18F-FDG could be a suitable endpoint biomarker.
Results: Preclinical FDG-PET imaging in xenograft mouse models of cancer showed a significant reduction in FDG uptake in tumour cell lines 786 and U87 (both PTEN nulls) versus the PTEN avid cell line BT474C. The FDG-PET data correlated with other PD endpoints such as the tumour size and protein biomarkers.
Conclusion: 18F-FDG PET imaging can be used as a non-invasive pharmacodynamic biomarker for clinical studies with the drug.
Case study of preclinical imaging using high frequency ultrasound and PET
Aim and Methods: Using a FeCl3 rodent model to induce thrombus formation on exposed femoral veins in mice, the hind limb functional flow with velocity 3D vasculature and time to occlusion will be measured. High frequency ultrasound can generate a 3D representation of the vasculature at baseline after injury and with drug treatment. PET whole body fibrin binding using a novel PET tracer (fibrin binding peptide label with fluorine-18) will be studied.
Results: Evaluation following different treatments are underway.
Imaging in clinical trials
Imaging is routinely used in clinical trials e.g. PET, CT, and MRI. Objective tumour response using the RESIST criteria is an example of an oncology biomarker.
Computed tomography (CT) and magnetic resonance imaging (MRI) are used to measure lesion size, detect appearance of new lesions, and provide anatomical information. Advanced imaging techniques such as PET, SPECT, dynamic contrast enhanced MRI, and perfusion CT can provide further information on anatomical structure of tumour sizes and functional information such as metabolic activity, expression of molecular targets, and cell proliferation.
Case study of imaging PD markers in metastatic colorectal cancer
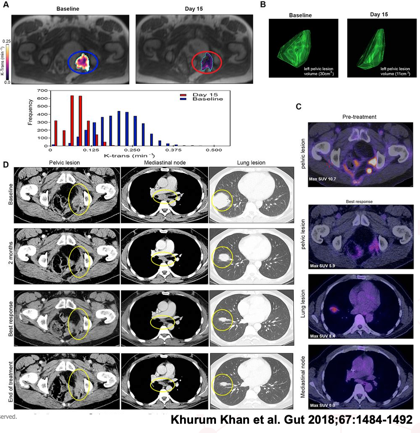
Methods: Imaging was performed at baseline and followed up at various time points. CT was used to assess volume change in tumour size, using the RESIST criteria and FDG-PET to assess metabolic activity of the tumour. Permeability and flow in the tumour and angiogenesis were assessed using dynamic contrast enhanced MRI.
Results: Reduction in permeability and flow in the tumour were observed (A) and tumour volume (B). FDG PET showed a decrease in metabolic activity (C) and CT images measured by the RESIST criteria showed a partial response to the drug for 31 weeks (D).
Conclusion: The imaging endpoints correlate with improved progression-free survival and overall survival in patients with metastatic disease in these trials.
This article is based on Neill’s talk from the MDC Connects webinar series. Watch the session Neill took part in – Understanding the PK / PD Relationship:

About the author
Neill Gingles has spent over 25 years working in academia, pharmaceutical and biotech SME industries. He has experience working in various therapy areas such as infectious disease, cardiovascular disease and oncology from preclinical early development through to late stage and marketed products. He has recently joined MDC in November 2019 within the preclinical imaging team.