The MRC Regulatory Support Centre have developed The Health Data Access Tool Kit to help health researchers access NHS data.
Programme Manager, Alex Bailey explains the thinking behind it.
The researcher’s problem: I can’t get hold of the data I want
We are all governed by a surfeit of laws, regulations and policies in our everyday lives. Most of us manage to negotiate these restrictions so that we can get what we want out of life, and not end up in court. However, when it comes to accessing health data for research (in particular, NHS data), we are met with a seemingly impenetrable wall of rules, and intense scrutiny, that sometimes leaves us feeling like we are in court. The system can seem disproportionate to the risks.
Talking to the relevant stakeholders reveals that there are common issues that slow or prevent access to NHS data for research:
- Misunderstanding the law; especially the difference between the common law duty of confidentiality and data protection
- The same word and phrases meaning different things to different parties e.g. ‘commercial research’
- A failure to understand that data always exists within a context i.e. data identifiability is always dependent on both content and context
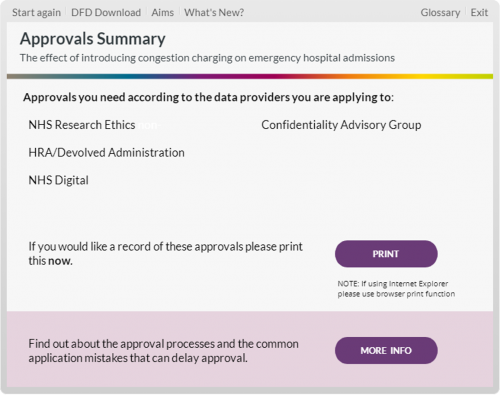
- Difficulties in knowing which research approvals are needed (especially UK-wide), including the difference between approval to access data and approval for research
- Lack of transparency about what research approvers and data providers base their decisions on i.e. what a good application actually looks like
The data provider’s response: Talk to us about it
We know that lots of researchers successfully obtain NHS data, and that data providers are very helpful in talking researchers through their own access systems. However, this support can be limited to their own processes and there is a general lack of expertise about the whole landscape. For example, a data provider can help you to use their application process for data that they hold, but they won’t be able to tell you if you need NHS research ethics approval or not. NHS research ethics committees will provide an opinion on your research, but they can’t tell you if it will satisfy the data provider. Also, although some approvals are UK-wide (e.g. NHS research ethics), there are a number of idiosyncrasies when gaining approvals or access to data from more than one UK country.
Our solution
To help, we have developed The Health Data Access Tool Kit. The Tool Kit is designed to guide researchers through the approval processes required to access routinely collected NHS data in the UK. It also provides the background to, and tips for addressing, issues specific to certain data providers and research approvers that are known to be rate-limiting steps. It was developed as part of NHS Digital’s Research Advisory Group in conjunction with major UK NHS data providers and the relevant research approvers.
What did we learn?
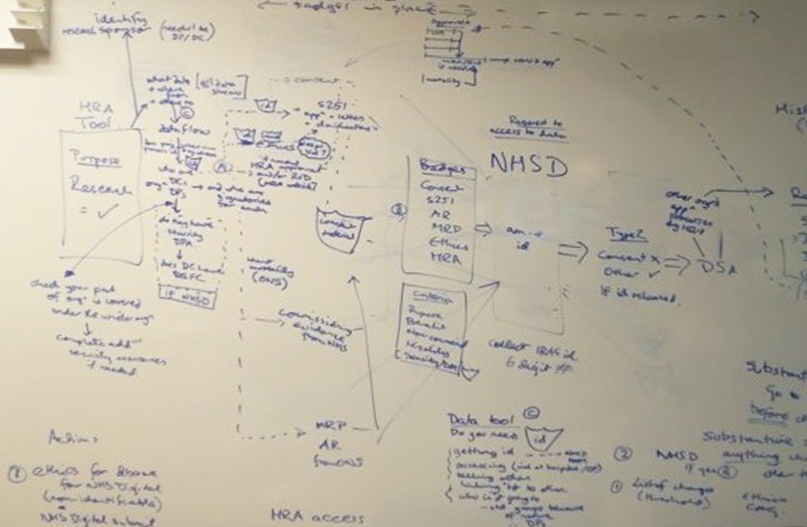
Unsurprisingly, developing the Tool Kit wasn’t easy. Even a single application process became complicated once every step was plotted out:
We were fortunate that we managed to get the right people in the room at the same time, but even then, gaining an understanding of why different approval processes had evolved the way they had wasn’t always clear. It did however, highlight the steps in the process that were least understood by researchers and therefore caused the most delays. It also placed the data providers’ processes within the context of other research approvals. Doing this enabled us to distil the key elements to include in the Tool Kit.
An additional benefit was that this process strengthened the communication between the data providers and research approvers, meaning that complex applications (those involving multiple data providers) are now more likely to be handled ‘behind the scenes’, rather than relying on the applicant as a go-between.
Next steps
The landscape clearly needs streamlining, and through partnership with HDRUK we are hopeful this will happen. In the short term, we know that the Tool Kit only applies to a handful of NHS data providers, yet there are lots of interesting datasets available elsewhere in the NHS and wider and there is increasing demand to link these. We are looking at how to incorporate more NHS, non-NHS and non-health data providers into the Tool Kit.
The Tool Kit relies on researchers knowing what data they would like to access and from where. We know that this isn’t easy, and that requests can change once researchers know more about the data (coverage, quality, linkage potential, etc.). HDRUK, in the first phase of their Innovation Gateway address this, so researchers can easily find out who holds what. We are working with them to incorporate and further develop, our Tool Kit into the next phase of Gateway. The aim being an end-to-end integrated data discovery, approval and access system.
About the author
Alex Bailey is a Programme Manager at the MRC Regulatory Support Centre. He has 25 years’ experience of working in academic and NHS research. Initially as a researcher, then managing European research networks, through to managing NHS Research Ethics Committees. He currently works with health data providers, approval bodies, regulatory authorities and research governance teams to help streamline access to health data for researchers.