Designing formulations for pre-clinical and early stage clinical studies
Quay Pharma are a contract development and clinical manufacturing organisation who provides expert services in the formulation of molecules for systemic and localised delivery.
The development pathway
- The active pharmaceutical ingredient (API) is supplied by the contract manufacturing organisation
- Quay Pharma formulate the API for preclinical studies to maximise dose and absorption in the animal models
- DMPK and toxicity evaluation are carried out by a CRO
- DMPK and toxicity study results guide the Quay Pharma clinical formulation development starting with pre-formulation studies
- Formulation development is based on data generated from the pre-formulation studies, to generate early prototypes
- A feasibility batch of the lead formulation is produced, followed by ICH stability prior to the actual GMP manufacture.
General considerations in pre-clinical formulation
The aim is to maximise dose and absorption, typically using a liquid dose and often with poorly water-soluble molecules with little API available. Excipients should be carefully considered due to potential side effects which can differ between animal species. Useful data to aid this process include chemical structure, solubility data, logP, pKa, melting point, Caco-2 data, amount of API available, maximum target dose required in the animal species and intended pre-clinical species.
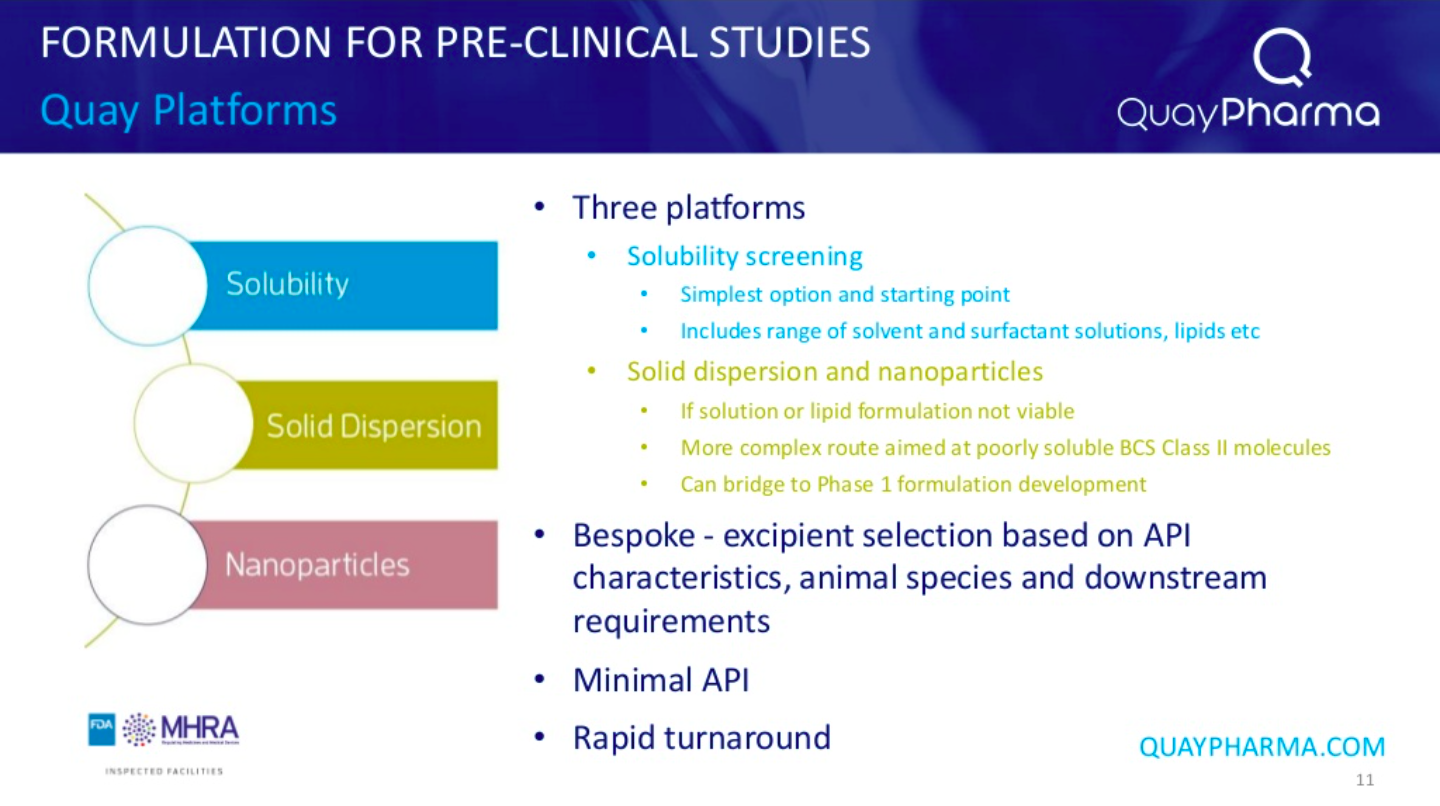
Quay Pharma utilise 3 main platforms for pre-clinical formulation, initially aiming for a simple solution formulation before investigating more complex formulations (solid dispersion or nanoparticles) if a simple solution is not possible.
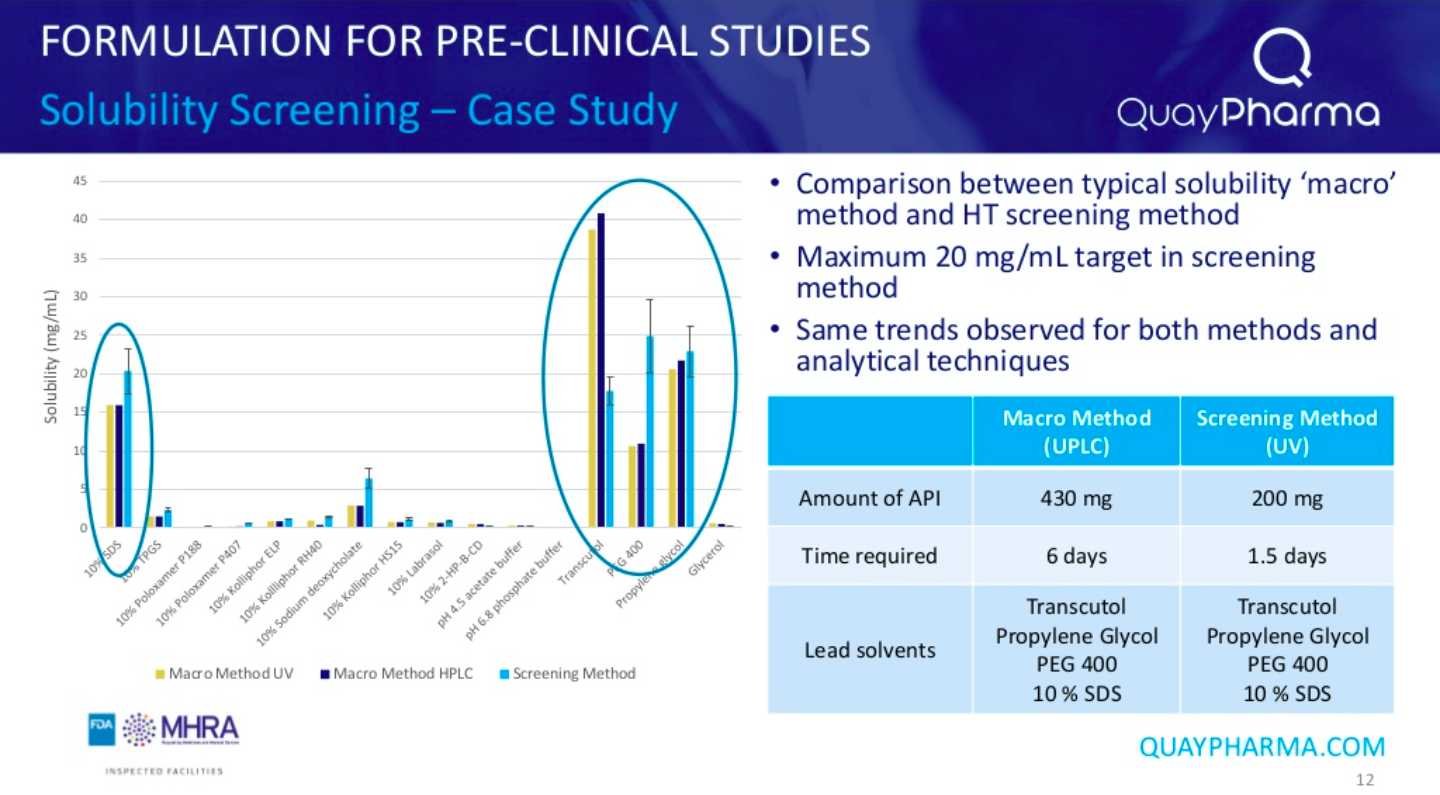
A comparison of a typical ‘macro’ equilibrium solubility screening method (UPLC) with Quay Pharma’s own screening method revealed similar trends for both methods and analytical techniques, however, the Quay Pharma method used 50% less API and took a quarter of the time.
General considerations in early clinical studies
Considerations include patient health benefits and requirements, the phase I study goal, preferred route of administration, target product profile, target release profile and target absorption site. The balance between the chance of success against the degree of acceptable risk and the balance of the costs and time to reach a phase I study is critical. Further consideration is a submission strategy, commercial considerations, API availability and finally the regulatory timeframe.
Prior to formulation, data required include the physicochemical characteristics of the API, particle size, distribution, shape or density, flow characteristics of the API, polymorphic form, and stability data. Biological characteristics include permeability, Caco-2 cell data, any potential efflux mechanisms, and the existing pharmacokinetic data.
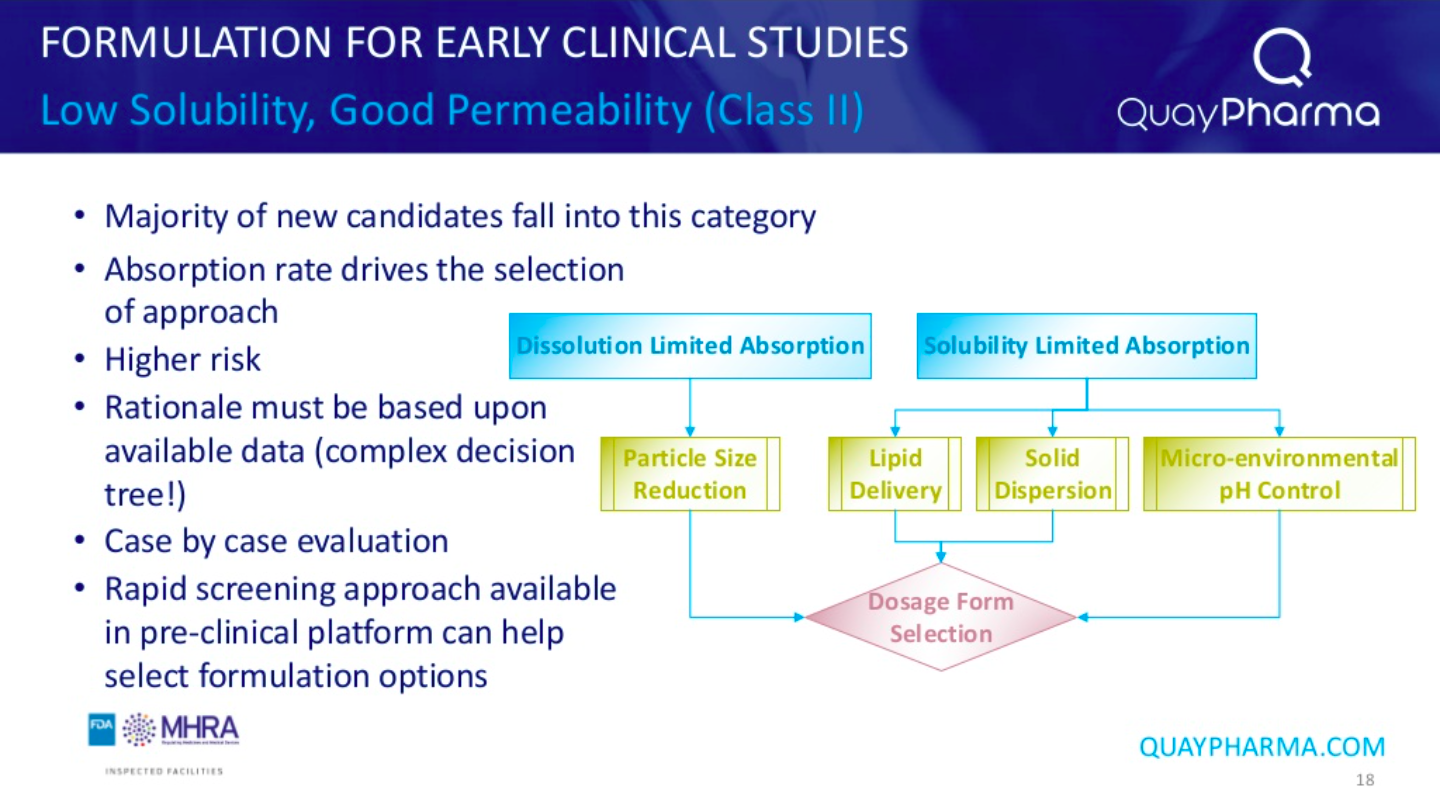
Data obtained from pre-clinical studies can help define the developability classification system (DCS) together with the target dose from the target product profile. Molecules fall into one of 4 categories–Class 1 which show good solubility and good permeability, class 2 and 3 which have poor solubility or permeability, respectively, and class 4 molecules which are difficult to formulate based on both poor solubility and permeability. Most compounds fall into the class 2 category.
Essential to the entire process is the balance of risk, time, and cost. An advanced formulation at phase I leads to an easier transition to subsequent phases but is likely to take longer to develop to meet the Phase I study timeline. Conversely, moving to a more complex formulation at a later stage (Phase II/III) may be a riskier approach as the complex formulation may lead to a difference in the bioavailability in man compared to the results obtained from the formulation used in the Phase I study.
This article is based on Alison’s talk from the MDC Connects webinar series. Watch the session Alison took part in –Chemistry and Formulation:

About the author
Alison Foster moved to Quay Pharma 7 years ago and is currently the Head of Technical for Pre-Clinical Services. Prior to this she was one of the founders and Pharmaceutical Programme Director of a small nanotechnology start-up company focussed on improving bioavailable of poorly soluble drugs. She has a wide breadth of experience having worked previously for Unilever in their cross-category research unit focused on Oral Care and Hair projects. Alison gained Post-Doctoral experience in medicinal chemistry from the School of Pharmacy, University of Manchester following a Ph.D. in synthetic chemistry.