Axis Bioservices is a preclinical contract research organisation based in Northern Ireland, providing services in oncology, inflammation, and respiratory disease. The company specialise in in vitro efficacy and mechanistic studies through to in vivo target engagement.
Value of PK-PD Studies to Maximise Pre-Clinical Efficacy
Preclinical pharmacokinetic-pharmacodynamic (PK-PD) analysis looking at dose-response relationships of exposure and biological effect both in the plasma and target tissue can enhance the drug discovery process. Benefits include predicting time course effects of drug doses (single or multiple doses) and pharmacological effects and provide an understanding of target modulation and the dosing schedules required to lead to statistically significant efficacy whilst minimising toxicity.
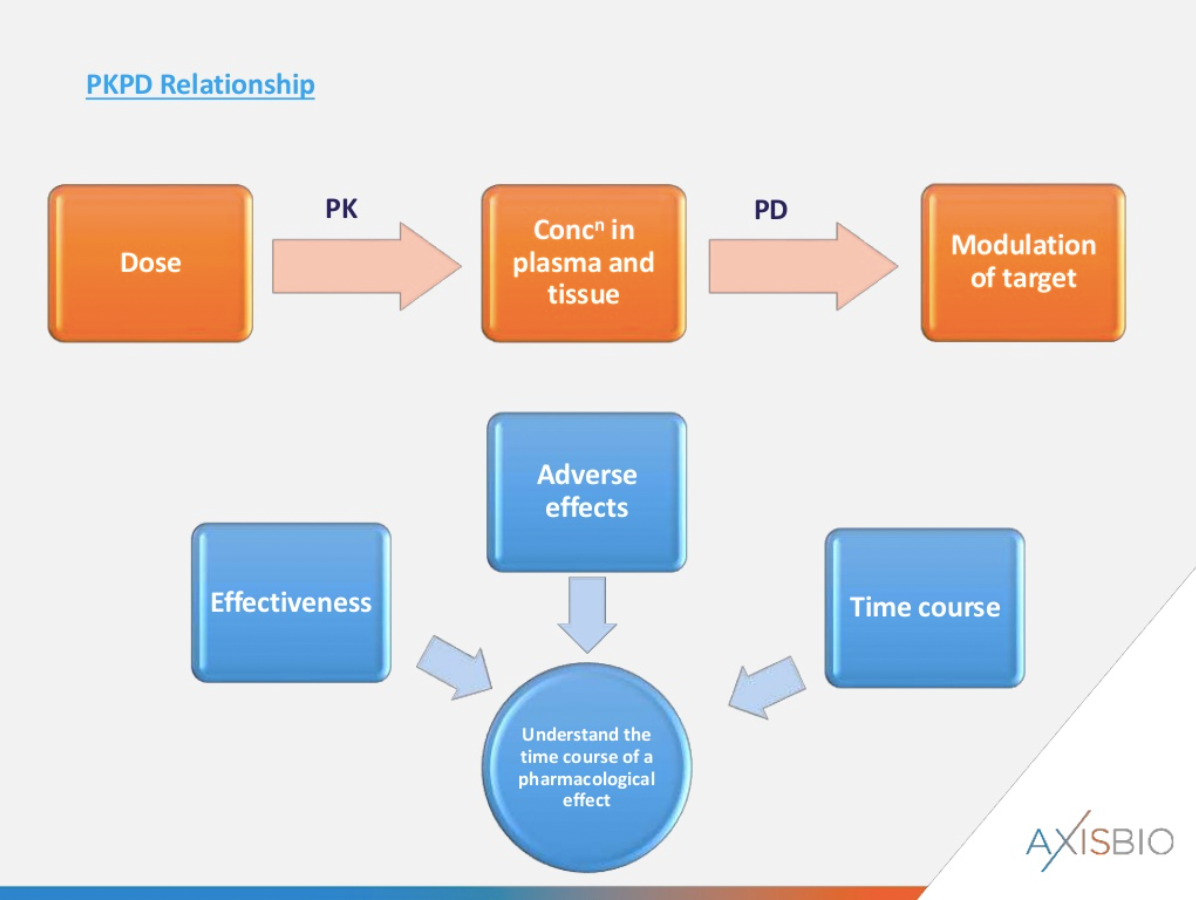
The PK-PD Relationship
The exposure levels of a drug following dosing in both plasma and target tissue can be quantified using PK analysis. PD analysis studies how the drug is acting at the site of action and helps answer questions such as does it modulate the target? What happens downstream of the target? Are there biological effects/efficacy?
Analysing a range of doses is key, alongside looking at effectiveness over time. In addition, it may be possible to correlate the PK-PD parameters with any adverse effects seen.
Integration of Knowledge
Knowledge integration is critical to design a successful PK-PD study and should combine the knowledge of chemists, pharmacologists, and biologists.
A successful PK-PD study should include:
- A range of doses e.g. 3
- A range of time points e.g. 5–6 around Tmax
- A washout phase to assess direct or indirect effects on the target
- Measurement of plasma levels, and target tissue
- Multiple samples from individual animals
Demonstrating the Benefit of PK-PD Models Prior to Efficacy Studies
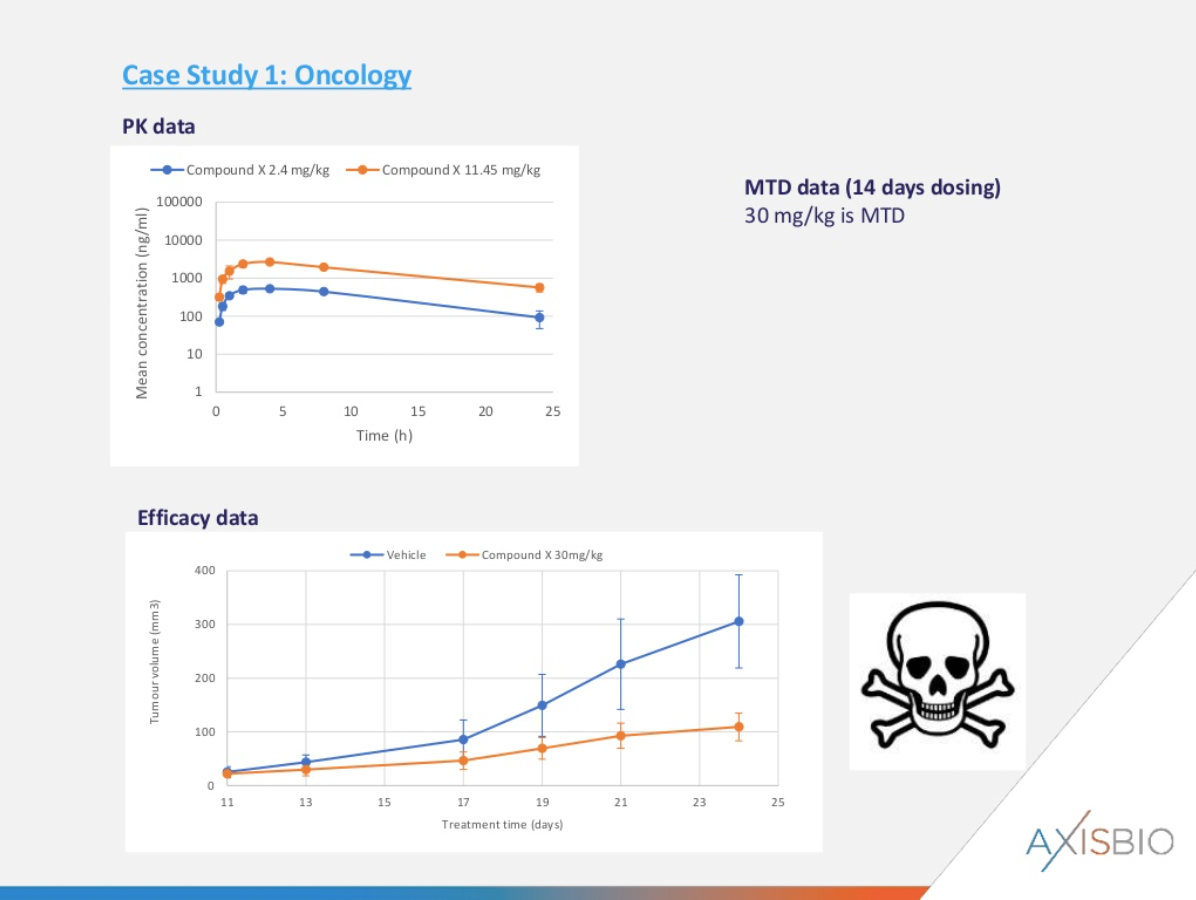
Case study 1 – no PK-PD analysis
Problem: Following plasma PK analysis, the drug moved directly to an MTD efficacy study with a 30 mg/kg dose. Whilst efficacy was apparent, tolerability was an issue leading to early termination of the study.
Solution: Axis Bioservices designed a single-dose PK-PD study at three dose levels (3, 10 and 30 mg/kg) with longer time points compared to the original PK study. Serum exposure levels, tumour exposure levels and both tumour target protein levels by Western blot and the associated downstream processes were analysed.
Outcome: Exposure levels were very similar at 10 and 30 mg/kgs, as was target protein modulation. However, indirect effects were apparent downstream. The results led to a change in the dosing schedule from 30 mg/kg once daily to 10 mgs/kg 3 times per week to give good efficacy and acceptable toxicity. Conducting these studies prior to animal models would have saved time and budget.
Case study 2 – PK-PD analysis
Respiratory studies can take 6 months, so problems encountered can impact on timelines.
PK-PD study design: Previously obtained PK data from naïve and diseased animals, in vitro data, and modelling data were used to design a PK-PD study with a 14-day optimised dosing schedule.
Results: The study design led to a successful efficacy study with full-scale biomarker analysis. This has allowed the client to proceed to the clinic with confidence in the dosing schedule.
Planning a PK-PD study provides the opportunity to maximise efficacy studies in the short term and plan for long-term success.
This article is based on Jenny’s talk from the MDC Connects webinar series. Watch the session Jenny took part in – Understanding the PK / PD Relationship:

About the author
Dr Jenny Worthington is Co-Founder and Director of Science at Axis Bio. She gained her PhD from the University of Ulster, Northern Ireland, in the area of cancer gene therapy, and worked through postdoctoral positions before establishing a research team in prostate cancer preclinical research. Jenny has used her background in cancer research and drug discovery to move into a commercial setting. Using her knowledge and experience she has nurtured excellence in a team of scientists who provide clients with an excellent preclinical service portfolio.