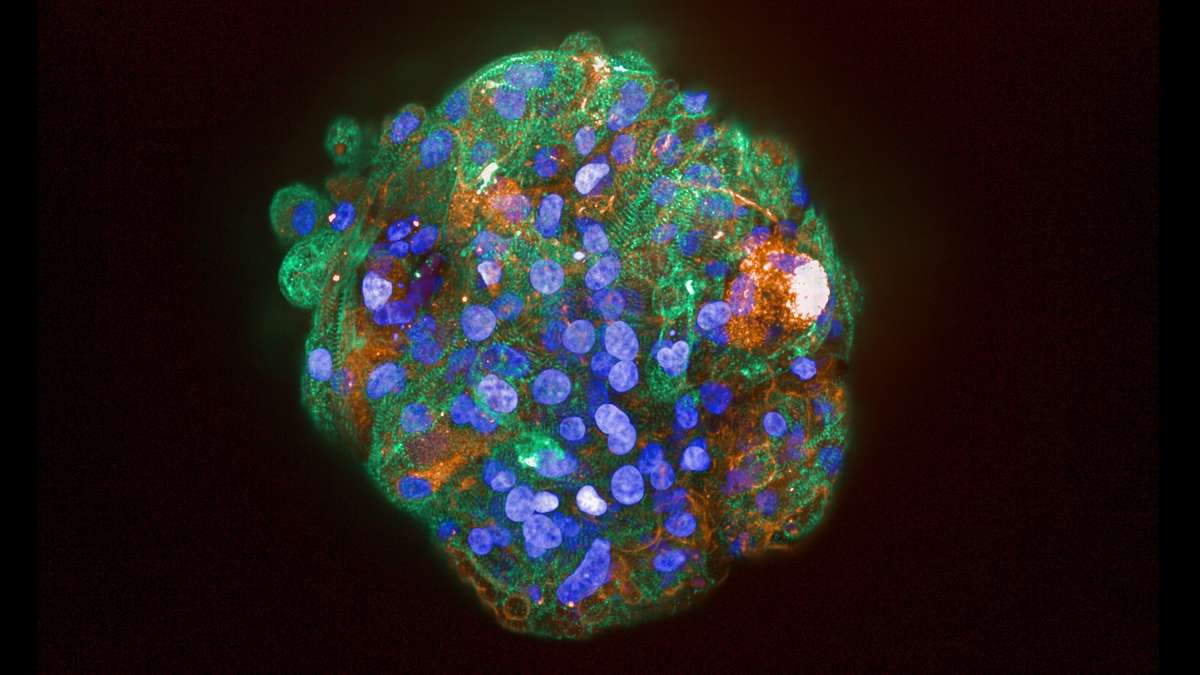
Clinical attrition due to lack of clinical efficacy during development remains an issue and can in part be attributed to inadequate pre-clinical target validation and/or suitable clinical translation data. Using relevant preclinical cell-based models can help ensure the clinical validity of the target & therapeutic effects prior to clinical efficacy studies and ensure a better understanding of complex biology and complex diseases.
Prior to assay development, it is important to consider the type of model required and whether it can adequately reflect the complexity of the disease. There should be a good understanding of the actionable data required, how to characterise the relevant biology in the model and therefore decide which is the most suitable model approach for the study. Sometimes a simple model that faithfully recapitulates a key functional requirement may be suitably fit-for-purpose. Sometimes a more complex model may be needed. The main consideration is that the model best reflects the biology required.
Advantages and disadvantages of primary cell models
The use of primary cells models has advantages, disadvantages and some opportunities. Primary cells have the advantage that they are native cells, the use of which enable a better understanding of the native physiological, morphological and molecular processes in human cells and are therefore provide the most in vivo-like cellular biology possible.
The disadvantages of using primary cells include the routine availability and sourcing of cells, the need for regular access, the scale/numbers required for higher throughput screening models and therefore the need to often source cells from individual donors resulting in interindividual variation within the assay and the challenge of maintaining the cellular phenotypes in culture. Whilst interindividual variation can be a disadvantage for certain applications (e.g. screening), it can also provide an opportunity, as it ultimately better represents what variation may be observed in the clinic.
The key opportunities with primary cells is the increasing ability to perform / access high volume isolations for certain cell types e.g. PBMCs which can undergo cryopreservation for future studies and provide consistency across multiple assays. Also, the ongoing evolution of more sophisticated, multicellular culture platforms offering longevity of relevant function.
Primary cell-based assays are typically positioned at the tertiary screening or candidate selection stage. However, appropriately validated primary cell-based assays can also be used to generate bioequivalence data to support marketing authorisation applications. Ultimately the choice of model and the positioning of the assay and relevant clinical translation of biomarkers carried through to clinical development need to be considered within the preclinical development cascade.
Primary cell assays can be useful to explain the pharmacology and show differences between primary cells and other cell lines. The use of primary cellular models can allow not only support validation of the target and therapeutic efficacy but also demonstrate the potential for any safety issues. It’s equally important to appropriately model both the efficacy and disposition / safety characteristics of a novel drug.
Summary
Clinical translation of the model is key to its success. Modelling of native biology, at native receptors in simple or complex systems, is possible. For it to be a success the positioning needs to be considered – the format, complexity, different cell types, the throughput required, and the source materials. The model must then characterise as fit for purpose and to ensure it can generate the data required.
This article is based on Amanda’s talk from the MDC Connects webinar series. Watch the session Amanda took part in – Target Validation and Efficacy:

About the author
Amanda Woodrooffe has 25 years’ experience in drug discovery and in vitro ADME gained from her roles in the biopharma and CRO industries. She has been responsible for operations management and scientific leadership of the UK-based discovery research services business for Asterand / BioIVT since 2010, This business has very recently been acquired by Precision for Medicine, becoming their 2nd European laboratory operation. Prior to this, Amanda held various positions responsible for developing business to business partnerships, pharmaceutical licensing, patent portfolio management and supporting corporate development. Amanda received her PhD from the University of Cambridge.