CEO, Chris Molloy, attended the Delivering novel therapies in the 21st century meeting at The Royal Society from 24 – 25 October 2018. Here, he shares his key learnings from the event and what they mean for helping the drug discovery community deliver.
Drug discovery is still failing too often: new treatment types are outdating the ‘white pill once per day’. Instead data is in the driving seat; it’s the most exciting time in history to be in medicines discovery.
Despite the many treatments we have today we are still scratching the surface of disease and new modes of therapy. We have a long way to go to really target addressable patient subgroups with any precision, particularly outside of cancer. If this sounds depressing, it should not. This conference was about delivering, not despairing. It’s the most exciting time in history to be in medicines discovery.
A 21st century approach became clear and simple: if we can clearly identify a patient-associated biological target – particularly one that spans multiple disease pathologies – and use patient-relevant systems to model them, we will then use each new weapon in an increasingly sophisticated and broad therapeutic arsenal to attack it.

The problem (better) understood
Drug discovery fails 80-90% of the time. The conference agreed that our lack of understanding in disease biology leads us to make drug candidates which are increasingly safe, but do not work in patients.
Mene Pangelos showed how AstraZeneca has reduced their failure rate to 80% but believes that it should be driven down to 60-70%, balancing productivity with the right level of risk. Genetics plays a big role and is a language we understand at a basic level. However, we need to learn the nuances of it. Even where we do, there is a complicated path from the gene through ‘real’ biology that is not well understood.
Professor Charles Swanton at the Francis Crick Institute (and founder of Achilles Therapeutics), showed how cancers evolve to treatment, and how these genetic changes can be used to define combinations of existing drugs that will treat that patients’ cancer.
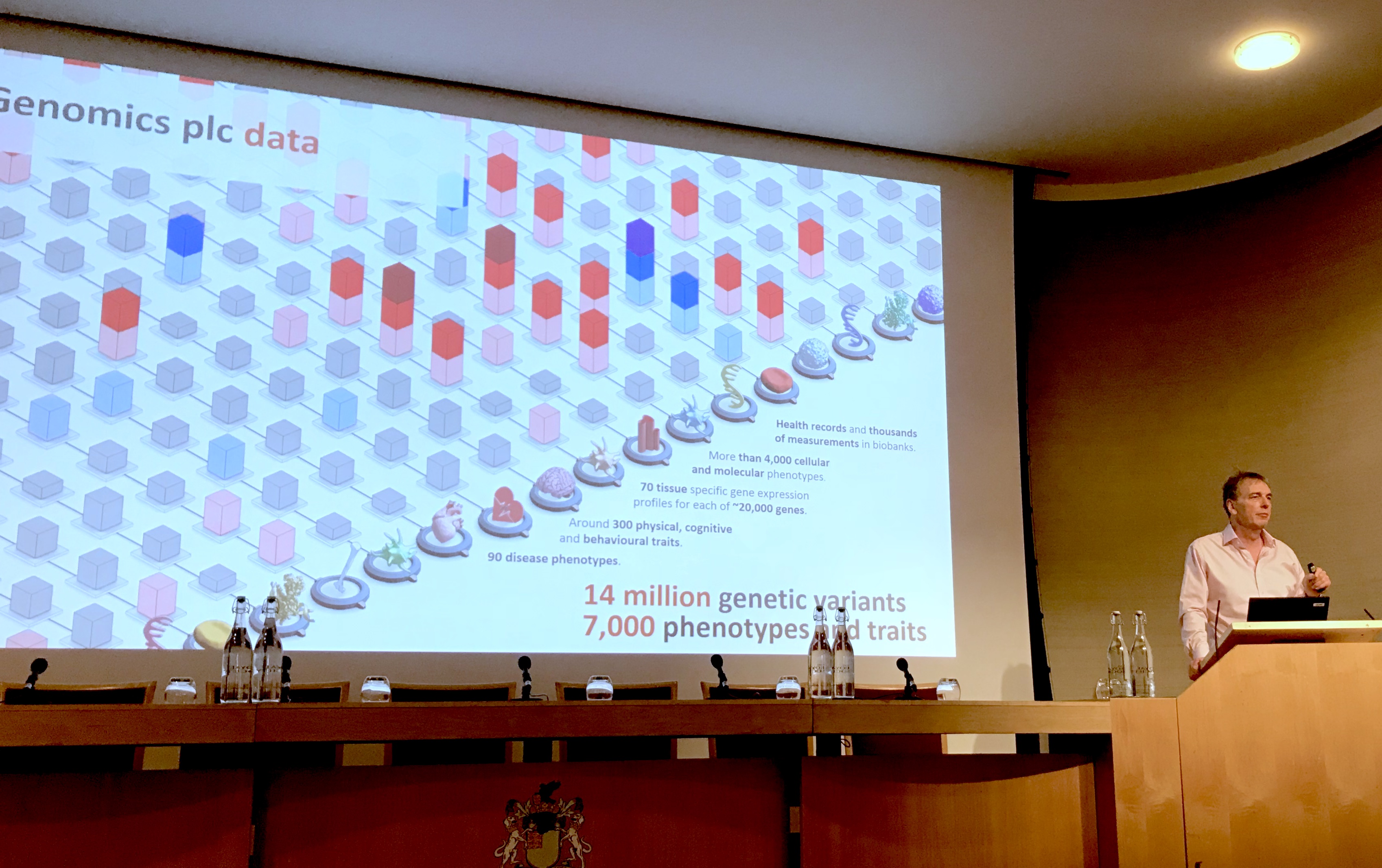
Professor Peter Donnelly at Genomics plc described how Mendelian randomisation in the ‘normal’ population has already performed many experiments that mimic the effects of future medicines, which gives us enormous insight into their potential, or not!
The tools we should use
The types of medicines we give to patients will be across a much wider spectrum of ‘modalities’; small molecules, large molecules, RNA, DNA, cells and ways in which we can harness our cellular machinery to attack disease elements themselves.
Tony Wood at GSK and Jane Osbourn at MedImmune showed how a wide array of new techniques like PROTAC, and even those we believe we know about – antibodies – have untapped potential.
Bent Jacobsen at Immunocore showed how targeted cell therapies have changed the future of oncology care and will do so in other diseases. They have also re-awakened the importance of effective drug combinations. Even if the potential of gene editing tools such as CRISPR-Cas9 seem today beyond the horizon, there are a host of exciting, valid approaches right in front of us.
Advanced Delivery Systems
The way in which we can now deliver these types of drugs is also developing to enable all of these new approaches.
Professor Sarah Tabrisi at UCL and Ionis Pharmaceuticals showed how intrathecal injection of an antisense therapy has achieved a first for Huntingdon’s Disease, and offers huge opportunity for other CNS diseases.
Kristofer Famm at Galvani Bioelectronics showed how bioelectronics can be used as treatment for not just nervous, but also multiorgan autonomically influencable diseases.
Professor Constantine Coussios showed how ultrasound can work in tandem with new liposome, and other formulations, to target and cargo drugs to sites of action. These approaches represent an innovative way of overcoming barriers to making efficacious, but poorly diffusible compounds clinically useful. The means of manufacturing them is also changing, from an industry focussed on large chemical batches, to a broader one that embraces antibody, chemical and cellular manufacture. We are using synthetic biology to get organisms to manufacture chemicals and now to manufacture small batches of life saving autologous therapies.
An Elephant in the Room
Professor Iain Buchan at The University of Liverpool demonstrated, through data systems, the disconnected (disease-by-disease) approach that healthcare and medicines take to treatment. He promoted a multi-agent AI approach to help understand the holistic ‘biology’. However, there is no therapy equivalent. Surely, we need to develop medicines that address some of the biological pathways that link these disease clusters to discover medicines – or combinations – targeted at common multimorbidities.
What is the way forward to help the industry deliver?
- Connected data to drive better target association using patterns unveiled by AI.
- Patient-relevant preclinical models in new areas of CNS, blood brain barrier and inflammation, and biology that links disease clusters together.
- Patient samples and data to ensure that projects and models start in the right place: with the patient.
- Access to the assets that research charities have: patient need, trust and a wealth of patient-centred biology.
Medicines Discovery Catapult is well placed to industrialise and drive the adoption of these new technologies and approaches. We can provide the expertise, equipment and facilities to help those with proven science create products that the industry will use.
About the author
Chris Molloy was appointed CEO of the Medicines Discovery Catapult in 2016. He has over 25 years international Executive and Board experience across multiple areas of Medicines Discovery.