Incorporating Diversity in Cardiac Testing
Heart and circulatory diseases are the leading causes of death worldwide, and the race is on to develop treatments that can help people with these diseases.
While this is a focus of many drug discovery companies, they must first overcome a significant challenge: cardiotoxicity (toxicity of heart tissue). Many medicines show efficacy throughout development and appear safe in tissue models. However, they can go on to reveal undetected cardiotoxicity once they progress into clinical trials or launch onto the market.
It is important to note that the risk of death from heart disease differs by race and ethnicity, which means that patient gender, ethnicity, genetics and individual drug responses play their part in understanding how to develop medicines that work for individuals.
However, there is a significant gap in effective models and tests to predict cardiotoxicity that reflects the disparities observed in the clinic due to race and gender.
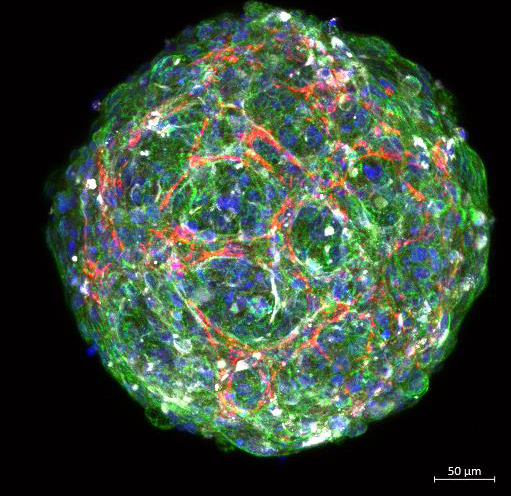
Edinburgh-based SME Cytochroma Ltd was established to address this lack of equality and diversity in drug development. It manufactures genetically diverse multicellular hepatic (liver) and cardiac (heart) models that better predict adverse reactions to medicines.
Cytochroma’s accurate preclinical models are being designed to predict toxicity earlier, reducing expensive late-stage failures while cutting time and costs, and accelerating more, safer medicines to market.
Read the Incorporating Diversity in Cardiac Testing Success Story here: