IP Protection Strategy: The University Technology Transfer Perspective
Dr Geraint Lewis, Head of Enterprise Services, Newcastle University
11 July 2024

This blog centres on the view of IP protection from a university technology transfer office (TTO) standpoint. It will highlight how TTOs typically assess IP, and how the process of securing the right protection also serves as a tool in refining the business model.
What Drives us to Seek IP Protection?
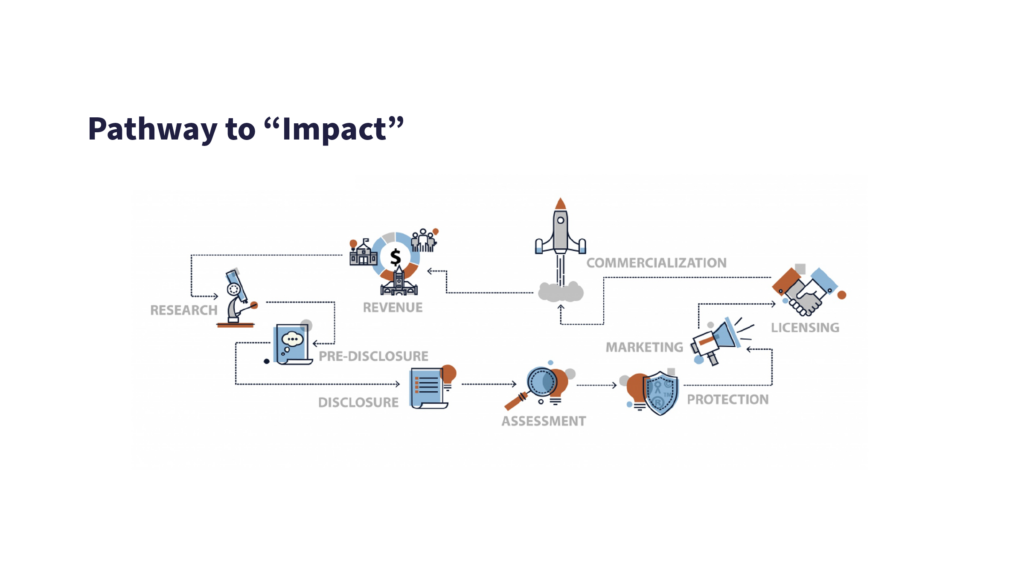
In common with most comparable university technology transfer services, Newcastle TTO’s brief is to create impact from research outputs. If the impact is best served by commercialisation, the IP involved will likely require protecting – whether it is for a university spin-out or a license to an established company.
In the majority of cases, the protection will take the form of patenting.
If the assessment stage reveals a viable opportunity with commercial potential it will be taken forward via a spin-out route, or licensing to an existing business. After patenting, marketing, licensing and commercial launch, the resulting share of revenue due to the university will eventually feed-back into funding its research projects.
The Assessment Stage: Developing an IP Strategy to Support the Business Model
This is the TTO’s decision-making process, ultimately with the focus on gaining a realistic vision of the invention’s ‘end-game’ and tailoring a plan to lead to that successful conclusion.
- By interrogating the business model, the product and the sector it will serve, a suitable IP and technology strategy can be formed.
- Equally important is researching the industry sector or sectors to be targeted and competitors in the same spaces. This helps highlight how the invention compares and what its value proposition is. It is also useful to identify other companies’ IP strategies – knowledge of what they are filing and where can be crucial to developing the best route to market.
- Assessment seeks to identify the IP assets most valuable to the business, and whether these can be patented – in terms of satisfying novel, inventive and freedom to operate criteria.
- The decision to patent key IP assets will consider the potential revenue each could generate through licensing or providing a competitive edge for a spin-out. Careful timing is also important; deciding when to seek IP protection should take account of any need for further technical development, and how that can be resourced.
What Happens when the University Files a Patent?
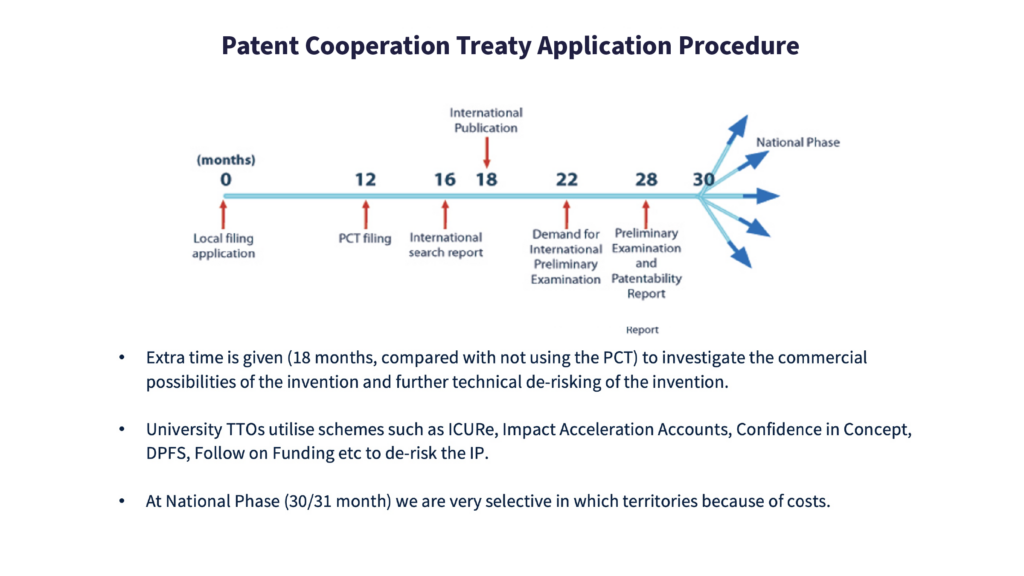
Following the Patent Co-operation Treaty (PCT) application procedure opens a window of 30 months to assemble all the data to understand the commercial possibilities of an invention in detail, and achieve further de-risking of the proposition.
This period is 18 months longer than the usual process, and allows scope for perfecting the invention’s market ‘fit’. The aim here is to maximise the opportunity’s attractiveness to potential licensees and funders, using the extra time to pull information together and to pursue a highly rigorous validation process.
Most university TTOs have access to some powerful tools – for work on proof-of-concept technical development, accelerating commercial de-risking and due diligence, for instance.
Selecting suitable geographic territories demands extremely careful consideration, primarily because the choices can be very expensive. Therefore, exhaustively researching the competition and opportunities in potential regions is highly important.
Again, discovering where competitors in the same field as yours are filing their patents can usefully inform the decision whether to enter a particular territory or opt for an alternative region.
Europe and the US are clearly prime territories to be considered, and China and India continue to grow in importance – both as markets and bases for manufacturing.
In Summary
The TTO’s over-arching intent is to closely align IP protection and management with carefully defined and relevant business objectives. The aim is to mould the invention into an ideal fit with the market, with all the relevant protections in place.
For most TTOs this is a very selective process – largely because of budget constraints. The imperative is to use resources cost-effectively to reach the point where licensing or spin-outs have the best chance of success. Value creation and capture are maximised when the right and necessary IP is protected.
Newcastle University’s TTO is a specialist in specific patent areas, in common with most UK universities. Much of the time the TTO works within one patent ‘family’.
It is worth re-emphasising the importance of talking to a TTO at an early stage. TTOs are often ‘blind-sided’ when patent filing is only considered after public disclosure has occurred, or when it is imminent.
This sometimes forces reactive rather than strategic patenting. The result is usually less well-tailored protection, and the opportunity to work-up an IP strategy over time for maximised market fit, attractiveness to backers and de-risking is often missed.
Finding and Accessing University Technology
Fostering personal contacts between university inventors and industry is an important role for TTOs.
Many strong, mutually beneficial relationships between academia and industry have been developed over the years, and they are often the source of successful technology transfers, usually involving an industry’s R&D teams and university personnel.
Making this connection – from either side – allows access to patent searches, useful publications and online marketing resources.
There are also several platforms specifically designed to bring inventors and industry together – IN-Part, Konfer and Leading Edge Only are three of the most popular.
Naturally, universities are ready to enable contacts through their websites. Newcastle University offers direct contact to or from the Technology Transfer Office here .
Dr Geraint Lewis, Head of Enterprise Services, Newcastle University
Much more detail is revealed by following this our fourth of five webinars in the series ‘Unlocking the Secrets of Medicine Commercialisation’ here.