Preclinical to clinical, building a translational link between in vitro and in vivo models of neuroinflammation for drug discovery.
Dr Emma Jones & Dr Lorna Fitzpatrick
September 2024
Neuroinflammation plays a key role in disease, injury, infection, and stress, with microglia, the immune cells of the central nervous system, being central to these processes. Central Nervous System (CNS) drug discovery from preclinical to clinical is complex, with a high failure rate often due to a lack of efficacy.
In this blog, Dr Emma Jones and Dr Lorna Fitzpatrick chart a course from preclinical to clinical. Reviewing a Medicines Discovery Catapult (MDC) project that demonstrates the power of using multiple models and technologies to readout neuroinflammation and improve the success rate.


The translation of therapies targeting Central Nervous System (CNS) disorders from preclinical drug discovery to clinical is complicated, with a high attrition rate often due to a lack of efficacy.
Identifying new therapeutics will require the evaluation of potential new disease pathways and different mechanisms of action to uncover novel disease targets. Neuroinflammation is proposed to play a major role across the spectrum of neurodegenerative diseases, and neuroinflammatory modulators are receiving increasing interest as potential drug targets.
In recent years, there has been a significant effort directed towards developing human in vitro CNS cell models to increase clinical translation. However, because the complexity and microenvironment of the brain are difficult to recapitulate in vitro, In vivo models also add significant value to the drug discovery process. Multiple models, together with innovative tools and biomarkers, are needed to expedite the development of novel therapies for neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease.
Neuroinflammation has been observed across all CNS pathologies, from neurodegeneration to stroke, psychiatric disorders and traumatic brain injury.
Microglia are the resident immune cells of the CNS and are key mediators of neuroinflammation. It has been shown that the production of proinflammatory cytokines by activated microglia, controlled by transcription factors such as NFĸB (nuclear factor (NF)- κB), is critical to disease progression. Inflammatory signals can also lead to inflammasome activation. The inflammasome is a multiprotein complex, and its upregulation is indicated by apoptosis-associated speck-like (ASC) puncta formation, induction of Caspase 1 activity followed by IL-1β maturation and secretion. Its role in neurodegeneration is increasingly recognised as a key indicator of the early stages of pathogenesis and is a potential target for novel therapeutics.
NFκB activation is also observed in microglia in response to stimuli in vivo. Moreover, translocator protein 18kDA (TSPO) is a widely used marker of activated microglia, expressed mainly on the outer mitochondrial membrane in response to injury. At MDC, we have developed the following models and tools to measure neuroinflammation and help our collaborators assess their novel therapeutics.
We have employed multiple techniques in vitro, in vivo and ex vivo to characterise acute neuroinflammation with multiple stimuli including immunofluorescence, advanced microscopy, plate-based assays, bioluminescence, positron emission tomography (PET), mass spectrometry imaging (MSI) and digital spatial profiling.
We have employed an NFKB pathway reporter (a) to monitor the induction of neuroinflammation in human iPSC-derived microglia in vitro. The lentiviral reporter drives expression of GFP and luciferase upon NFKB activation. This was measured live and we quantified neuroinflammation in response to a selection of inflammatory signals including lipopolysaccharide (LPS), β amyloid 1-42 and α- synuclein(b).
We showed that small molecule Inhibition of the NFKB pathway resulted in a reduced response to stimuli (c). (D) Transduced microglia in tricultures with astrocytes and neurons also responded to stimulation and inhibition of NFKB
Inflammasome activation occurs in two stages: priming (signal 1) and activation (signal 2). NFκB translocation to the nucleus to modulate transcription (as seen in Fig. 1) is a priming event and ASC puncta formation is signal 2, indicating inflammasome activation. ASC acts as an adaptor molecule facilitating the complex assembly resulting in Caspase 1 activation and IL-1β maturation and secretion.
We have developed a second fluorescent lentiviral reporter to track signal 2 induction following stimulation by Nigericin that can be analysed via either microscopy or plate-based assays. Pre-treatment with a small molecule (mcc950) inhibitor of the pathway effectively prevented inflammasome activation (Fig. 2). Together, these innovative tools provide an opportunity for novel drug discovery in vitro and to further understanding of microglia in neuroinflammation and CNS disease.
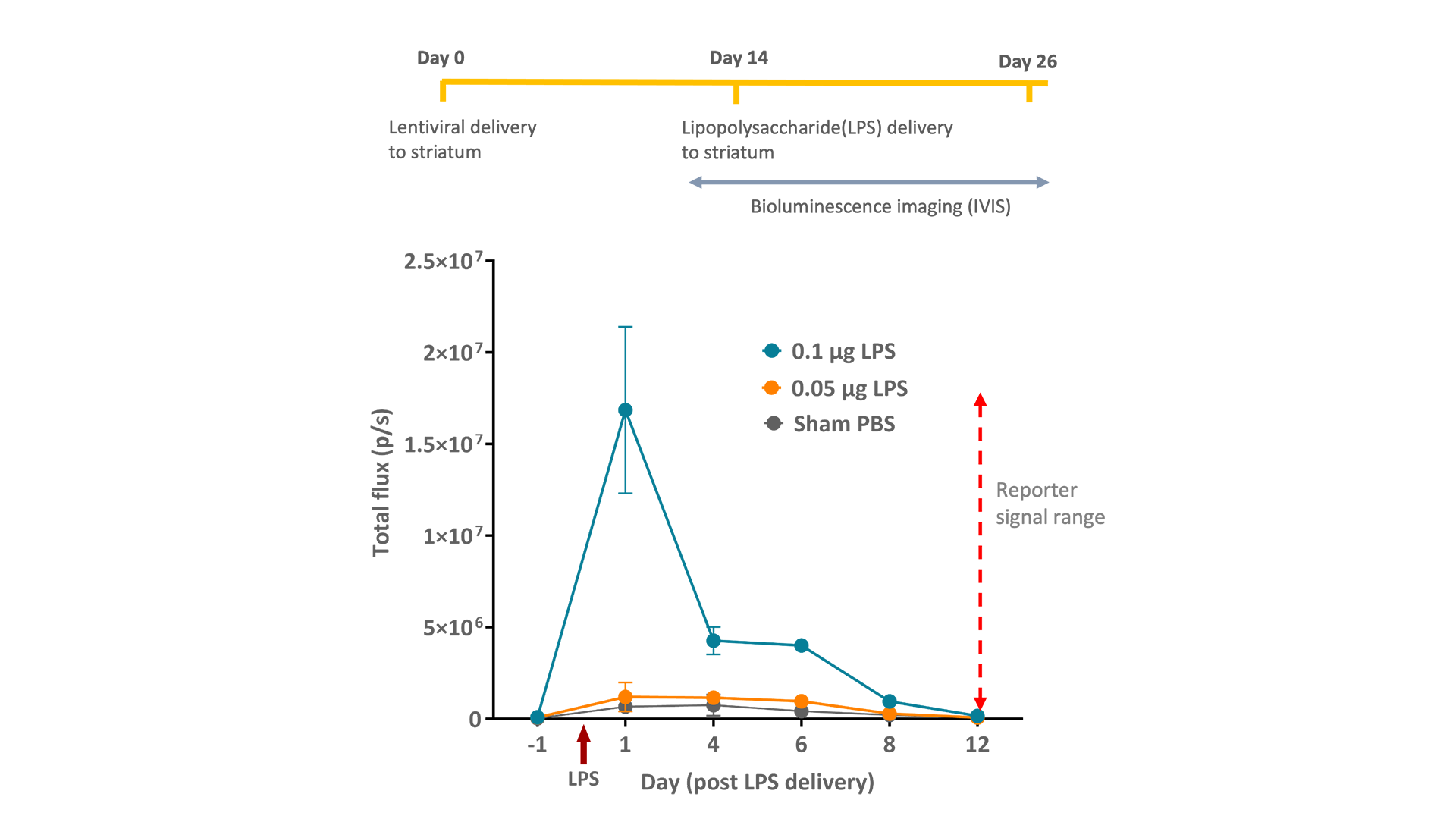
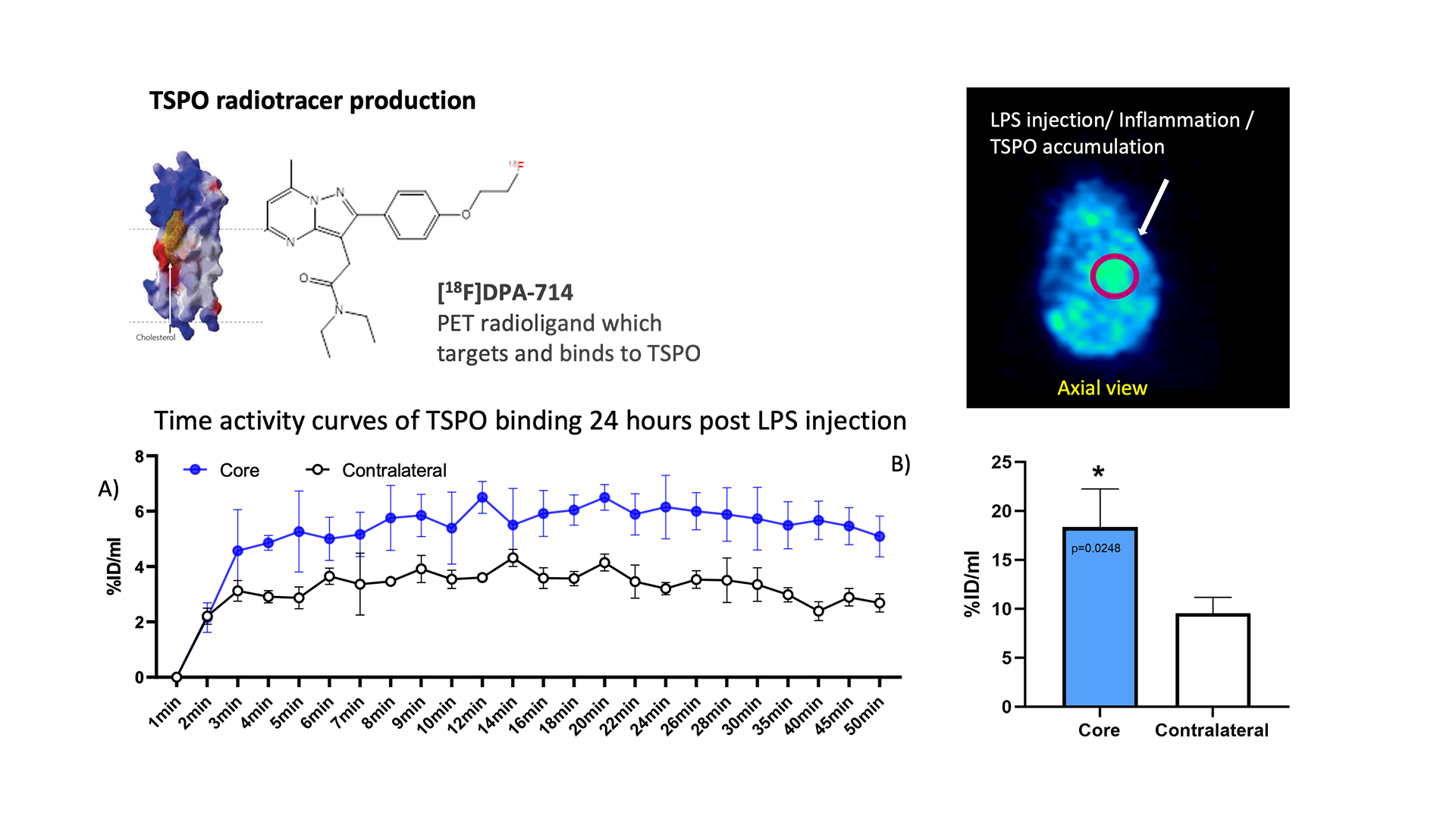
We have translated our NFκB lentiviral reporter from in vitro to in vivo to provide a live, real-time readout of inflammation activation following acute stimulation by LPS. We successfully delivered lentiviral particles to the striatum , one of multiple areas of pathology in neurodegenerative disease. The bioluminescent signal was measured on the intravital imaging system (IVIS) post LPS stimulation, demonstrating a marked increase in luciferase activity in the first 24hr (Fig. 3). In addition, we employed dynamic PET to monitor the onset of neuroinflammation in vivo, in real time. We saw rapid accumulation of TSPO radioligand ([18F]DPA-714 ) following LPS activation (24hr) in the striatum (Fig. 4).
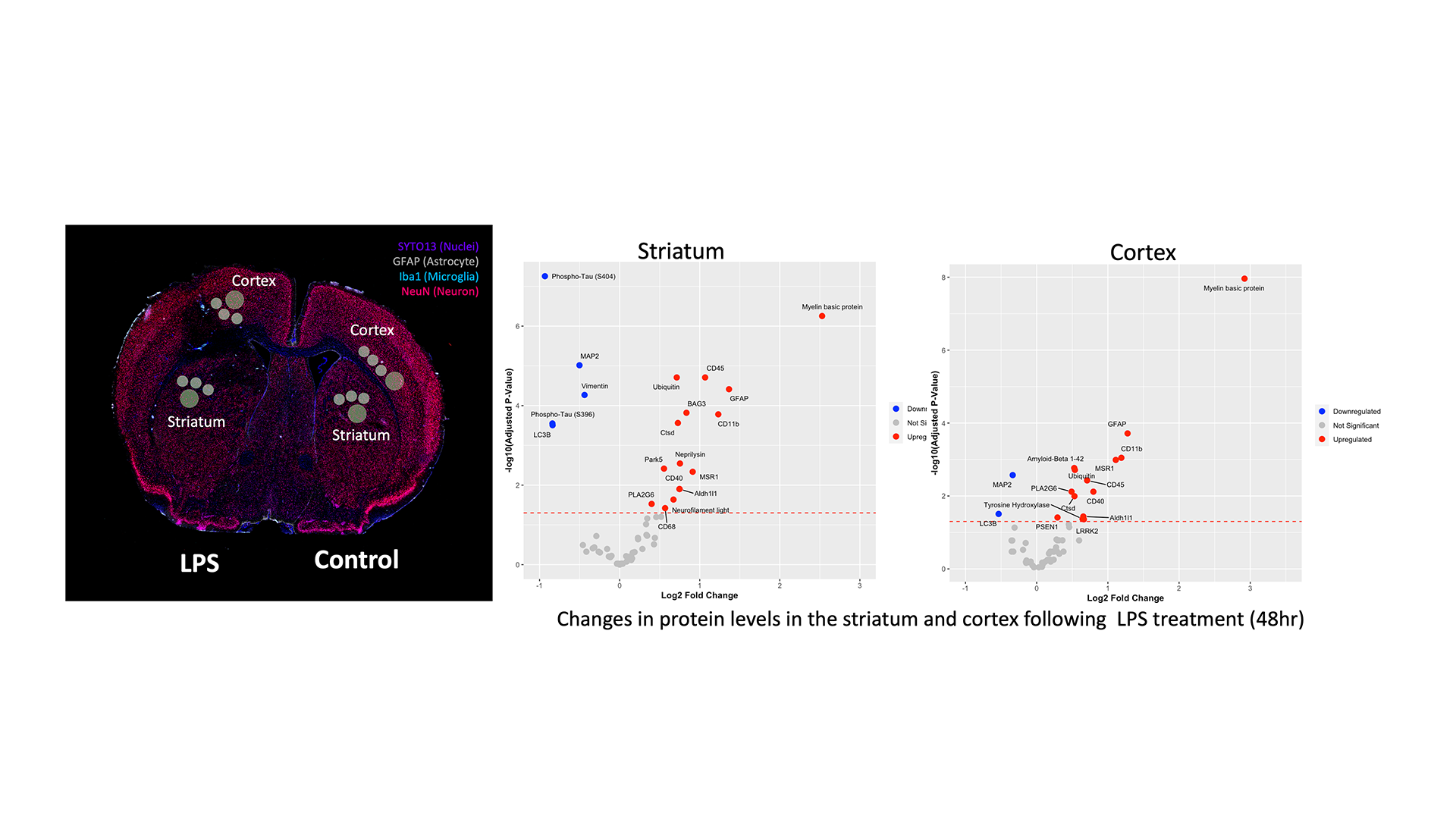
Immunofluorescence analysis of ex vivo tissue revealed that microglial activation and astrogliosis occurred at the site of LPS treatment. Little to no activation was observed in the contralateral region. Higher resolution imaging showed NFκB activity was present in all three cell types; neurons astrocytes and microglia (Fig. 5)
To understand the spatial transcriptomic changes that occurred in specific regions of the brain ex vivo, we performed digital spatial profiling (DSP). Our DSP platform enables the user to quantify molecular profile of cells within whole tissue sections or tissue microarrays. We found a significant increase in inflammatory genes in the striatal and cortical regions following LPS administration (Fig. 6).
Finally, we also performed complementary ex vivo mass spectrometry imaging (MSI). MSI is non-destructive and enables the molecular analysis of the tissue surface, each pixel generates a mass spectrum that contains information of a sample.
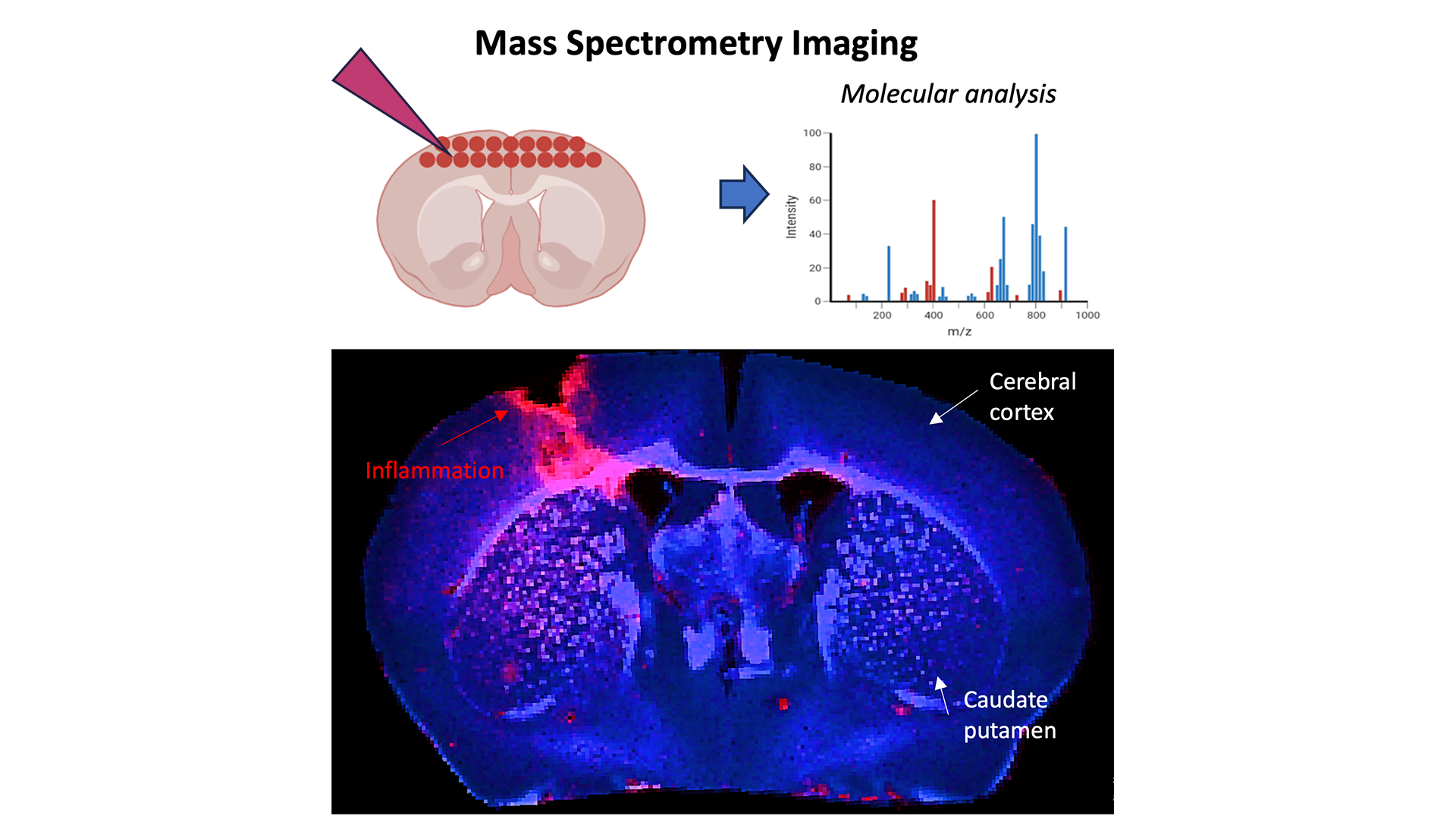
This project demonstrated the power of using multiple models and technologies to read out neuroinflammation. We hope to collaborate with partners to test their therapeutics or technology in some or all of our assays.