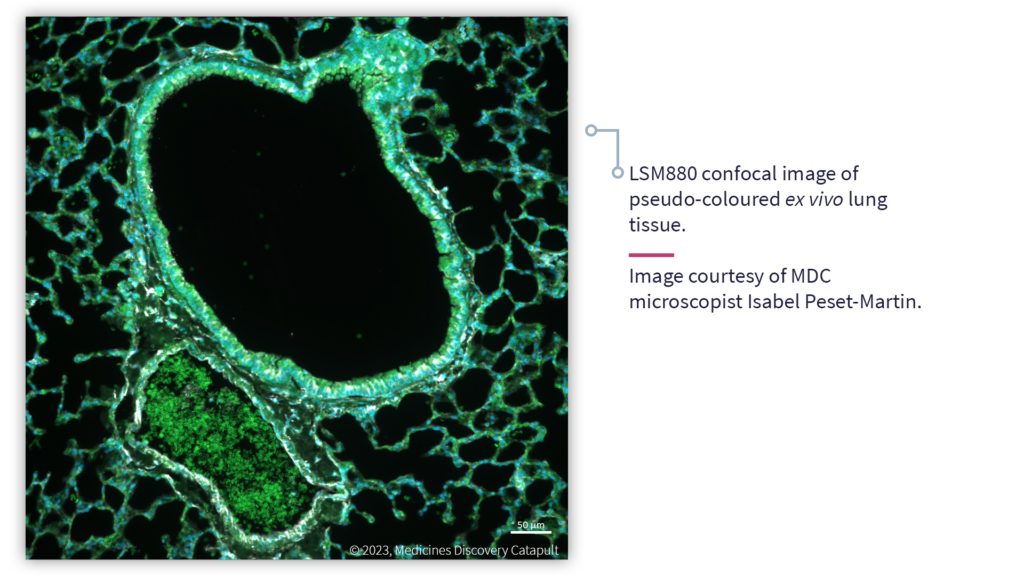
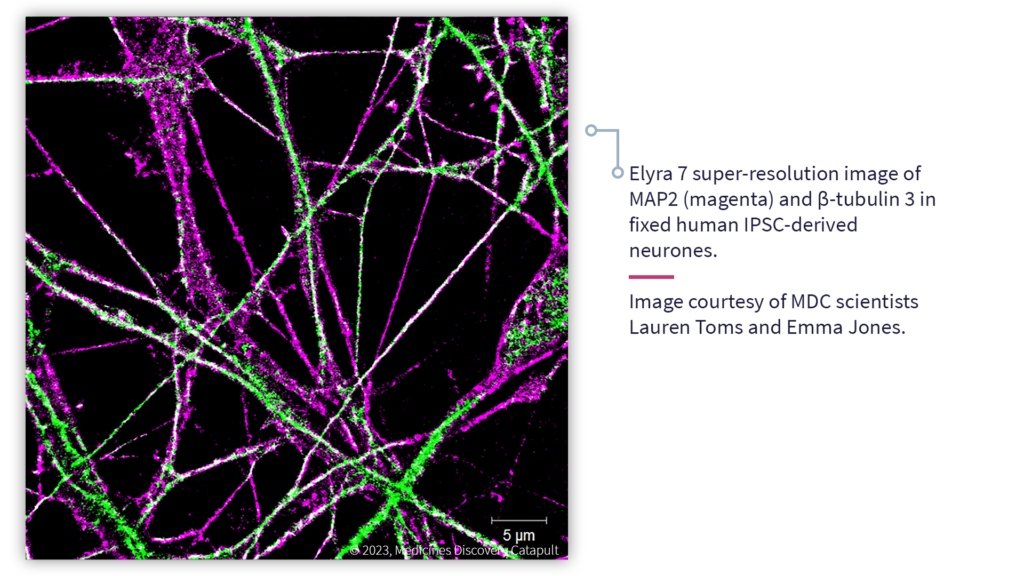
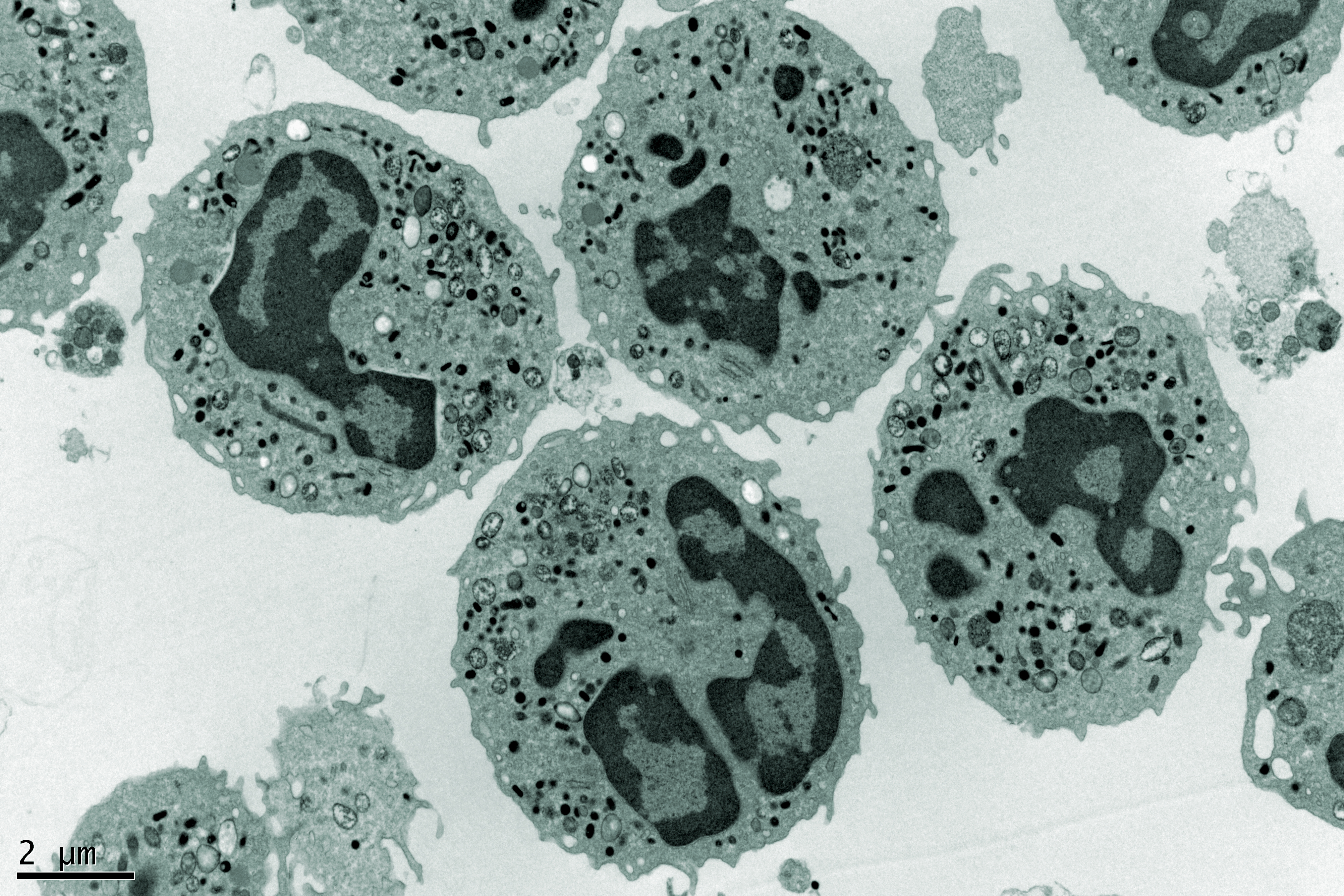
Advanced Microscopy enables the quantitative analysis of dynamic biological processes at scales ranging from single molecule interactions to complex multicellular structures and tissues
Visualising single cells and sub-cellular compartments supports medicines discovery by providing a non-destructive mechanistic assessment of your sample. For example, it can resolve drug-target engagement, understand pathway activation and evaluate phenotypic and functional responses. As a result, advanced microscopy technologies continue to revolutionise medicines discovery by providing novel insights and data that support therapeutic drug development.
We have experts in advanced fluorescence microscopy, and our scientists can draw on a diverse toolkit to characterise relationships between cell behaviours and underlying molecular processes. Due to its versatility and robustness, advanced microscopy is at the forefront of contemporary medicines discovery and is heavily integrated into our workflows in various projects.
Our Advanced Microscopy team brings together varied expertise to provide SME, academic and industrial partners with state-of-art imaging solutions.
Did You Know?
“Single molecular localisation microscopy can reconstruct a complete three-dimensional map of individual proteins in a single neuronal synapse.”
Challenges we can help with
We have a range of advanced microscopy specialisms that we can help the drug discovery community with:
How can you use Advanced Microscopy?
Fluorescence microscopy can be used to visualise tagged molecules, organelles and cells in cultured, or ex vivo, cell and tissue samples. Since the generated images directly represent target size, shape and abundance, fluorescence microscopy is highly quantitative.
By leveraging multiple fluorescence microscopy platforms, we can conduct studies of:
- 4D imaging (x,y,z & time)
- Mechanism of action
- Target engagement
- Subcellular distribution
- Cell-type identification
- High-throughput phenotypic screening
- Ex vivo tissue structure
- Drug phenotypic and functional response
- Analysis of 3D cell models
It is possible to multiplex tags, fluorescent labels and/or imaging modalities to build information-dense models. Such models provide detailed mechanistic insight at multiple scales and can be mined for novel features.
The breadth of the microscopy field and our varied instrumentation permits a bespoke approach to study design that’s directed by project requirements. We can determine the appropriate model system, imaging resolution, and phenotypic readout and then build a suitable imaging package.
Collaborating with our in-house informatics team, we can develop automated analysis pipelines for unbiased high-quality quantification. This approach ensures experimental validity and promotes robust decision making.
We leverage our expertise and imaging platforms to image cells on their terms, allowing us to provide partners with straightforward conclusions that inform their medicines discovery journey.
Come and speak to us, and see if we can help.

Dr Phil Auckland
Lead Scientist, Advanced MicroscopyExperimental capabilities
We have an established, well-equipped fluorescence microscopy facility capable of answering diverse experimental questions. This is supported by a dedicated group of imaging experts and a bioinformatics image analysis team.
Confocal microscopy with Airyscan and Multiphoton
The Zeiss LSM880 confocal microscope with Airyscan allows for high-resolution (to ~120nm laterally) 4D imaging of inter- and intra-cellular events while minimizing the damaging effects of laser exposure. The system is equipped with an incubator for live-cell work, assorted light sources for multiplexed imaging and an Airyscan fast mode for high-temporal resolution acquisition. The multiphoton upgrade allows for thick samples, for example, up to 0.5 mm tissue sections, to be imaged via a specialist excitation mode.
Super-resolution microscopy
Our Zeiss Elyra 7 system is tailored for a super-resolution approach known as single-molecule localisation microscopy (SMLM). Super-resolution imaging is a relatively new technology that can improve resolution up to 10-fold (to ~20 nm laterally) by using advanced optical and computational technologies. Using our SMLM pipeline it is possible to perform multiplexed imaging of single molecules and ultrastructural features in fixed cells and tissue sections. This is of particular importance for mechanism of action and target engagement studies.
High-content screening
Our Opera Phenix Plus high-content screening platform is based on a spinning-disk confocal system with ~250 nm lateral resolution. With simultaneous acquisition and an enclosed incubator, the Phenix is suited to fast imaging of fixed or live samples and can handle large-scale screens. Furthermore, our Phenix is equipped with automated plate and liquid handling that significantly improves reproducibility when conducting phenotypic screens, fast-response imaging and live-cell dosing experiments.
The advanced microscopy group prioritises adaptability and innovation when approaching problems. We are happy to work with collaborators to leverage our collective expertise and provide novel solutions to challenging questions.
Reshaping Medicines Discovery...
It's ambitious, it's achievable
-
For early-stage SMEs in this space, I thoroughly recommend engaging with MDC who have the end-to-end knowledge in complex medicines, can support your in-house team and provide access to key equipment that start-ups may not have.
Professor Helen McCarthy
Chief Executive Officer, pHion Therapeutics
-
By tapping into MDC’s unique drug development expertise and facilities, we are confident we will accelerate the preclinical development of our drug delivery system and its commercialisation.
Dr Anna Perdrix Rosell
Co-founder and Managing Director, Sixfold Bioscience
-
Working with MDC has provided expertise and intellectual input. It has also enabled access to a wider network of companies providing specialist services.
Dr David Templeton
Technical Director, N4 Pharma
-
It has been great to access the Artificial Intelligence expertise at Medicines Discovery Catapult, the team have taken an innovative approach that adds value to our product and will benefit our customers. Our collaboration was easy to establish and worked extremely well.
Phil Jones
CSO, BioAscent
Our Experts

Dr Phil Auckland
Lead Scientist

Dr Rebecca Kelly
Scientist

Dr Lauren Toms
Senior Scientist
Our Capabilities and Technologies
Speak to Us
We can help support you with your next drug discovery project.