We are at the forefront of biomarker discovery and validation. Integrating both standard and cutting-edge technologies to gain a comprehensive understanding of drug responses and disease mechanisms in the field of neurodegeneration.
The readouts from the various technologies can be interrogated either individually or overlayed with our team of data analysis scientists and bioinformaticians analysing and presenting the data in a clear and accessible format
We can examine either established biomarkers, including developing novel assays for specific molecules or perform hypothesis-free novel biomarker discovery. By discussing your project needs with you we can assist in building a bespoke plan to address any specific research questions you may have.
Liquid Samples: Cerebrospinal fluid (CSF) or plasma samples can be examined in single or multiplex to profile a range of different molecule classes from proteomics, nucleic acids, lipidomics and metabolomics.
Tissue Samples: Central nervous system (CNS) samples such as the brain and spinal cord can either be analysed whilst retaining the spatial information of the tissue or alternatively homogenised and analysed in bulk.
Exploring the Potential of Liquid Biopsies
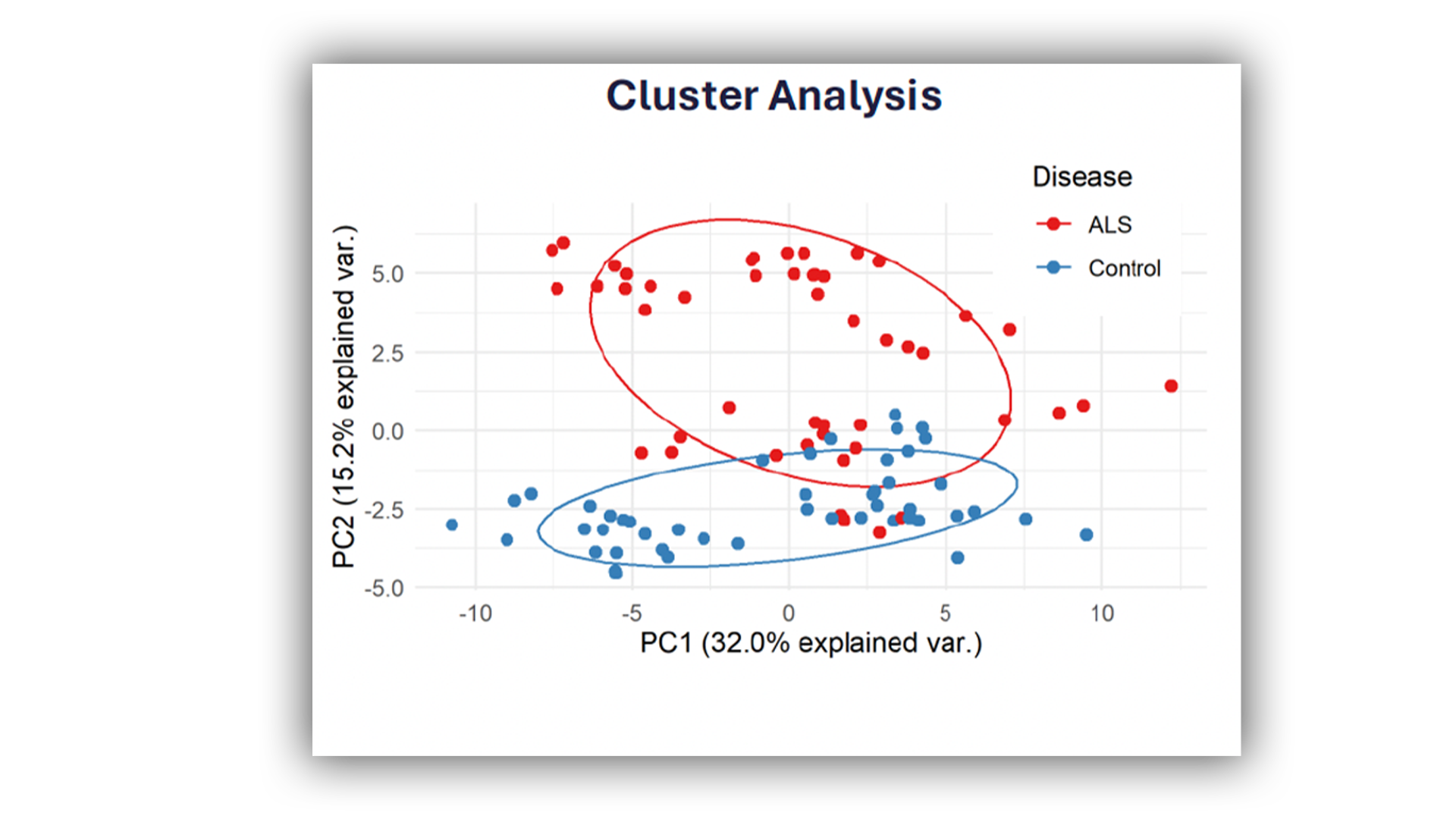
Liquid biopsies, primarily plasma and CSF samples within the field of neurodegeneration, offer a rich source of information for the identification of novel biomarkers and biomarker signatures. Harnessing the capabilities and expertise of MDC, we tap into a diverse array of biomarker classes, spanning proteomics, nucleic acids, lipidomics, and metabolomics, utilising various cutting-edge platforms and bespoke bioinformatics workflows for profiling and analysis.
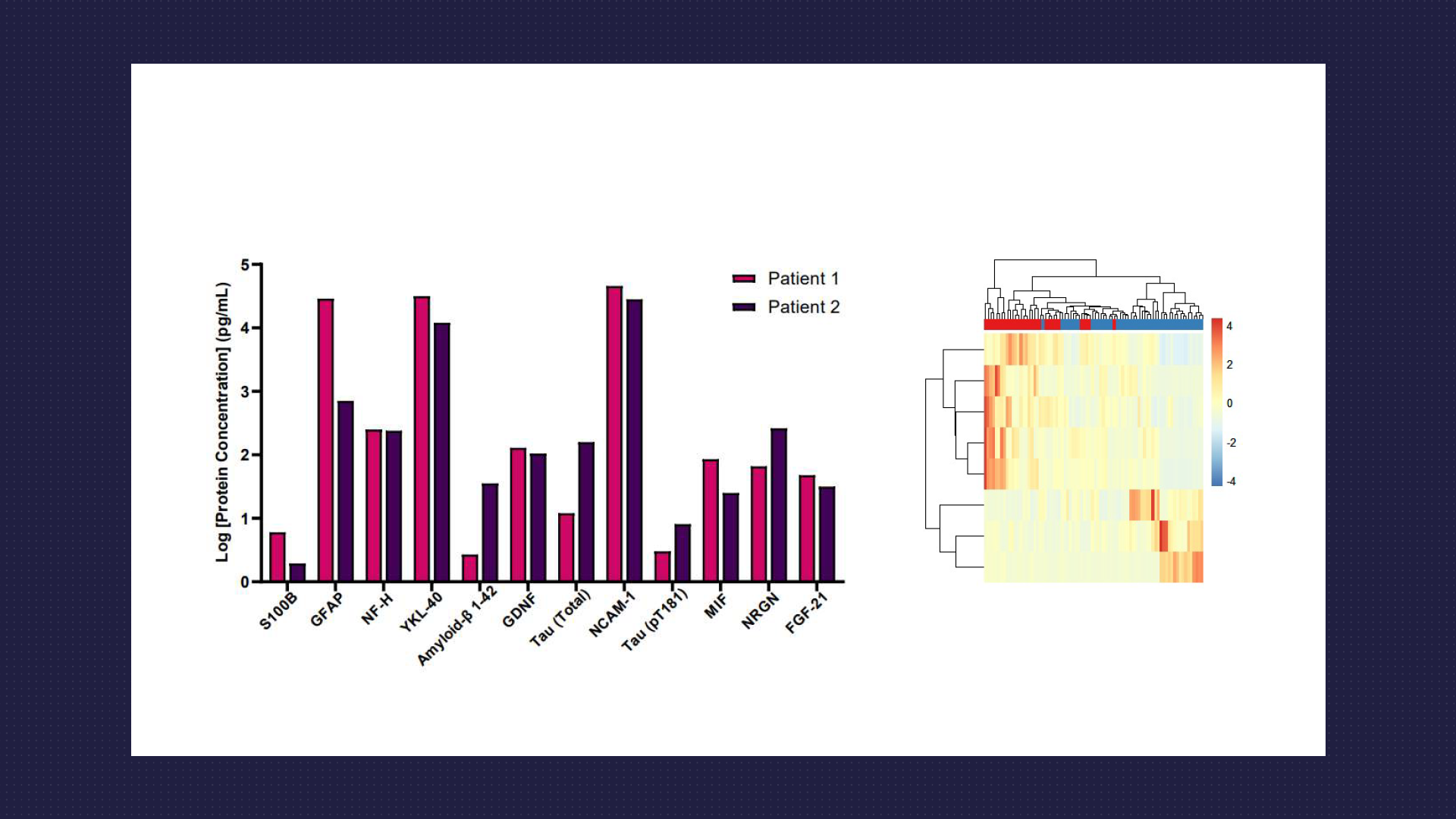
Targeted Multiplex Protein Analysis
-
- Utilise Luminex and Quanterix Simoa platforms for the routine multiplex detection of targeted protein panels. Our expertise extends to conducting multiplex assays on plasma and CSF samples, with a specific focus on proteins associated with neurodegeneration, neuroinflammation, and traumatic brain injury.
- Among the array of biomarkers measured, neurofilament proteins stand out as a crucial indicator of axonal injury and neurodegeneration.
- Assays cover a range of other key biomarkers, including but not limited to Amyloid Beta (1-40 and 1-42), Tau (total and various phosphorylated forms), GFAP, S100B, TDP-43, GDNF, BDNF and UCHL1. Our analyses offer invaluable insights tailored to your specific inquiries around biomarker research for neurodegenerative diseases.
- Whilst we run a comprehensive range of assays using commercially pre-validated assays, if an assay kit for a specific target of interest isn’t readily available, our experienced team has a track record of developing and validating customised assays in-house. These assays have been successfully multiplexed, enabling thorough screening of human plasma and CSF samples for your analyte of interest.
Untargeted Proteomics/ Lipidomics/ Metabolomics Analysis
In addition to the profiling of specific proteins the mass spectrometry team has extensive experience conducting untargeted analysis of a diverse range of molecules, successfully applying this approach to CSF and plasma samples.
-
- Proteomic analysis can examine the most abundant species, providing coverage for approximately 250-280 proteins. Additionally, it allows for profiling of individual amino acids, including their coverage and abundance.
- Metabolomics analysis can generate a set of untargeted data that would allow for a direct comparison between the comparator groups. When completed without a clear a set of targets, it will be a global comparison only.
- Lipidomics analysis can profile the most abundant lipids in the samples, including lipid global length and global saturation levels.
Circulating Nucleic Acids
- Exploring circulating nucleic acids presents a promising avenue for biomarker discovery.
- Our expertise includes profiling circulating mRNA as an indicator of transcriptome changes using patient plasma samples.
- Despite potential challenges such as low RNA yields, we have achieved notable success in this endeavour. Similar methods could potentially be applied to CSF samples for further exploration.
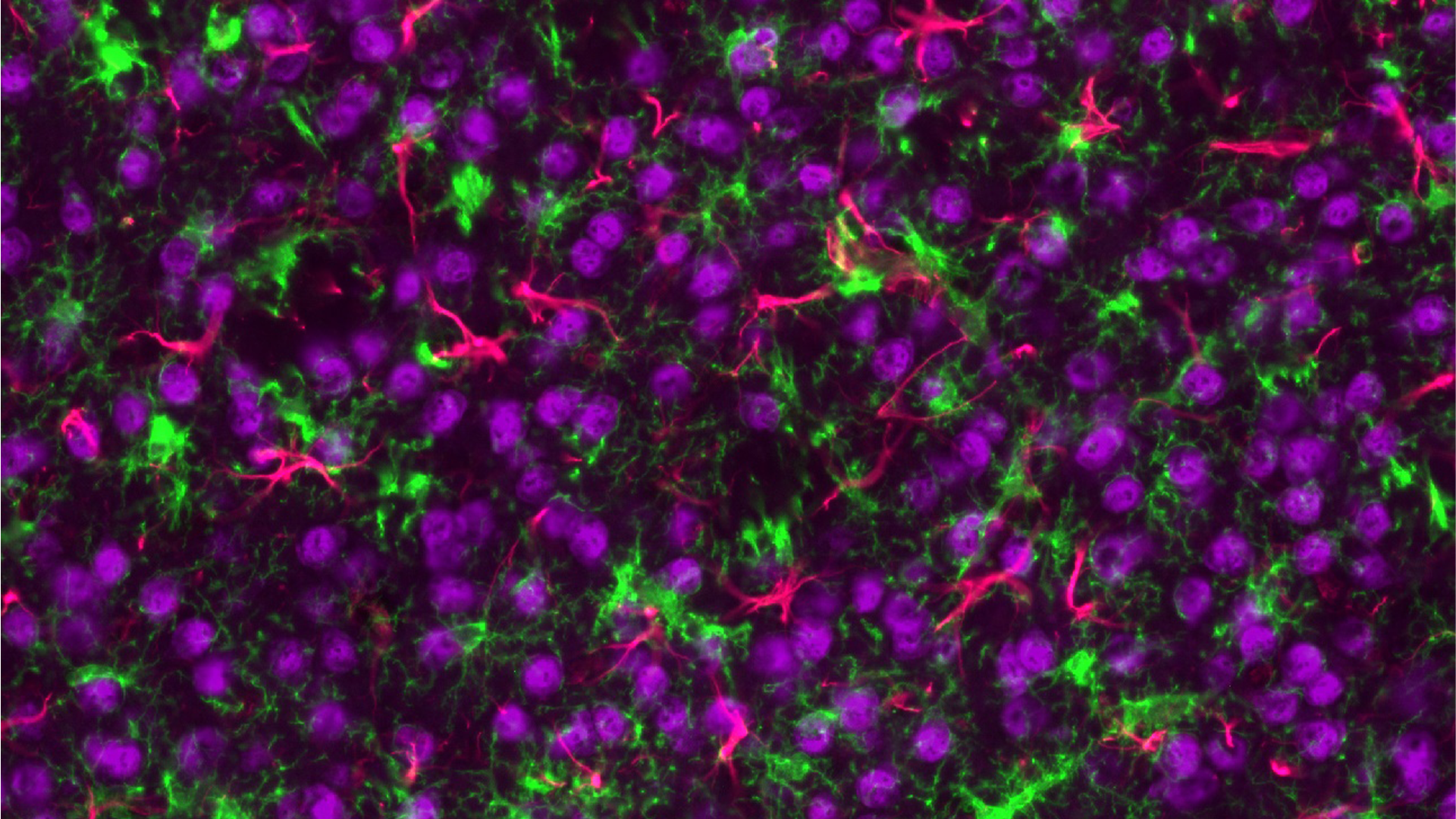
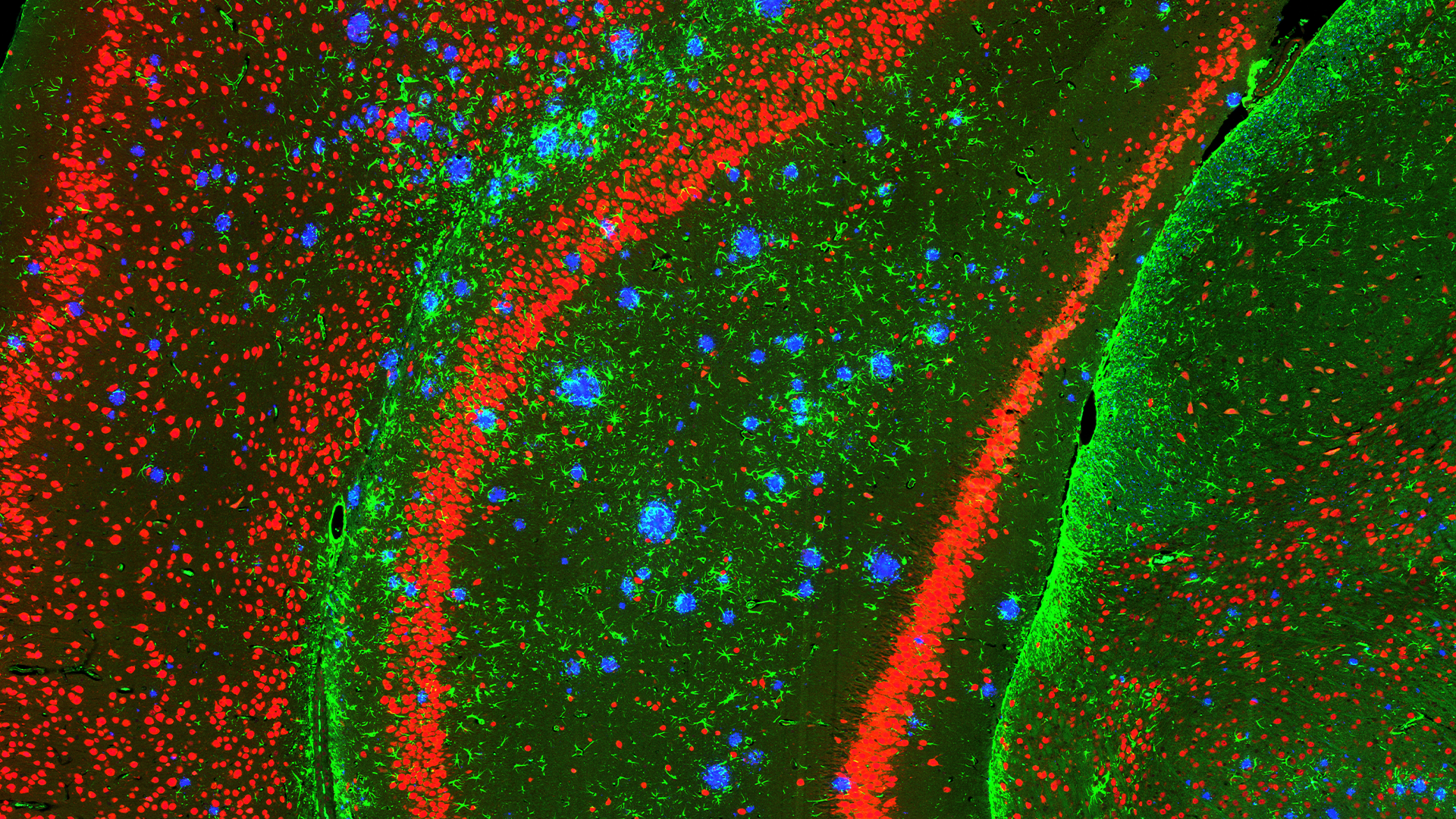
Exploring the Potential of Tissue Samples
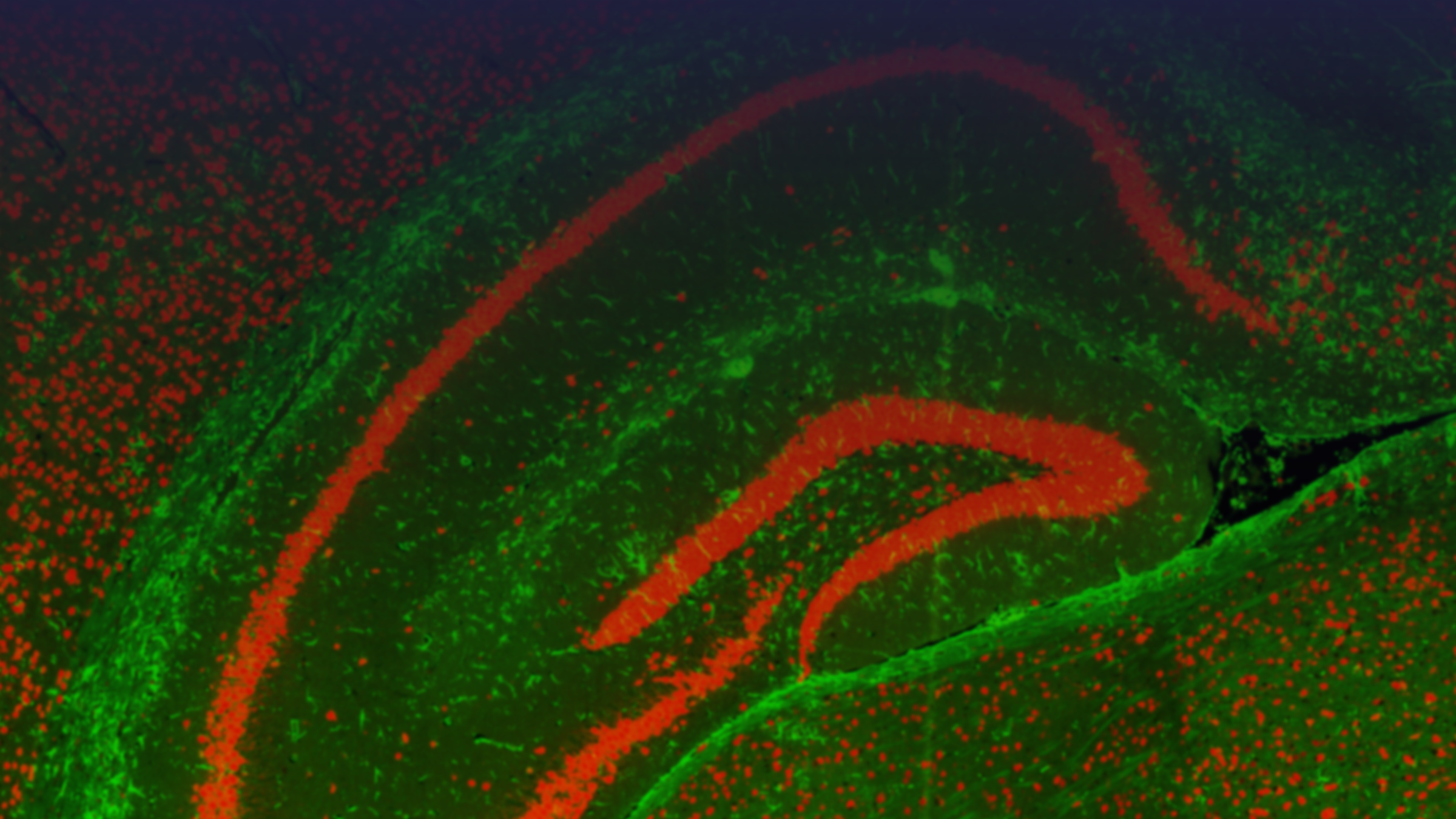
Tissue samples from human biopsies or in vivo models can be evaluated for a diverse range of biomarker classes (Proteomics, Transcriptomics, Lipidomics, Metabolomics) using an array of technologies. Common samples include human spinal cords and specific brain regions (e.g. cortex, hippocampus, striatum) or, in the case of in vivo models, whole brains and spinal cords. Depending on the specific research question samples can either be analysed in bulk or using methods that retain the spatial information of the tissue.
Bulk Analysis
-
- Homogenised tissues can be profiled using the range of techniques previously described for liquid biomarkers.
- Transcriptomics can also be examined in these samples, either whole transcriptome analysis or selected sets of about 800 genes using the nCounter platform.
- Whilst the spatial information is lost, using this approach can be more sensitive and is the best solution in some circumstances.
Spatial Analysis
-
- The spatial information can be retained for a range of biomarkers (Proteins, transcriptome, lipids, metabolites) and examined using the innovative platforms and techniques set up at MDC.
- Data from the different spatially resolved technologies, including more traditional immunohistochemistry analysis, can be combined both with other spatial technologies and either bulk tissue analysis or biofluids and overlaid for a more in-depth analysis of the tissue.
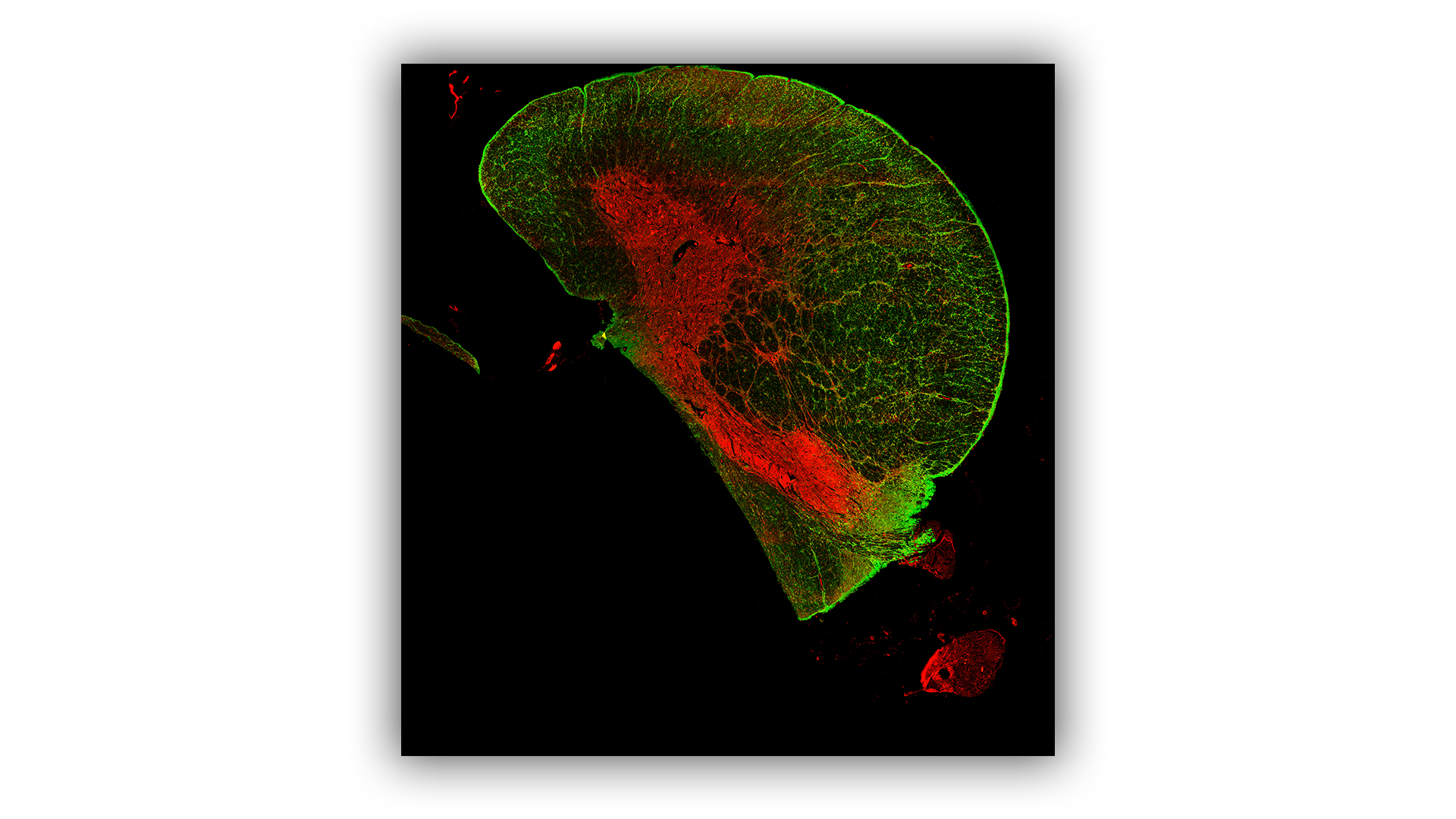
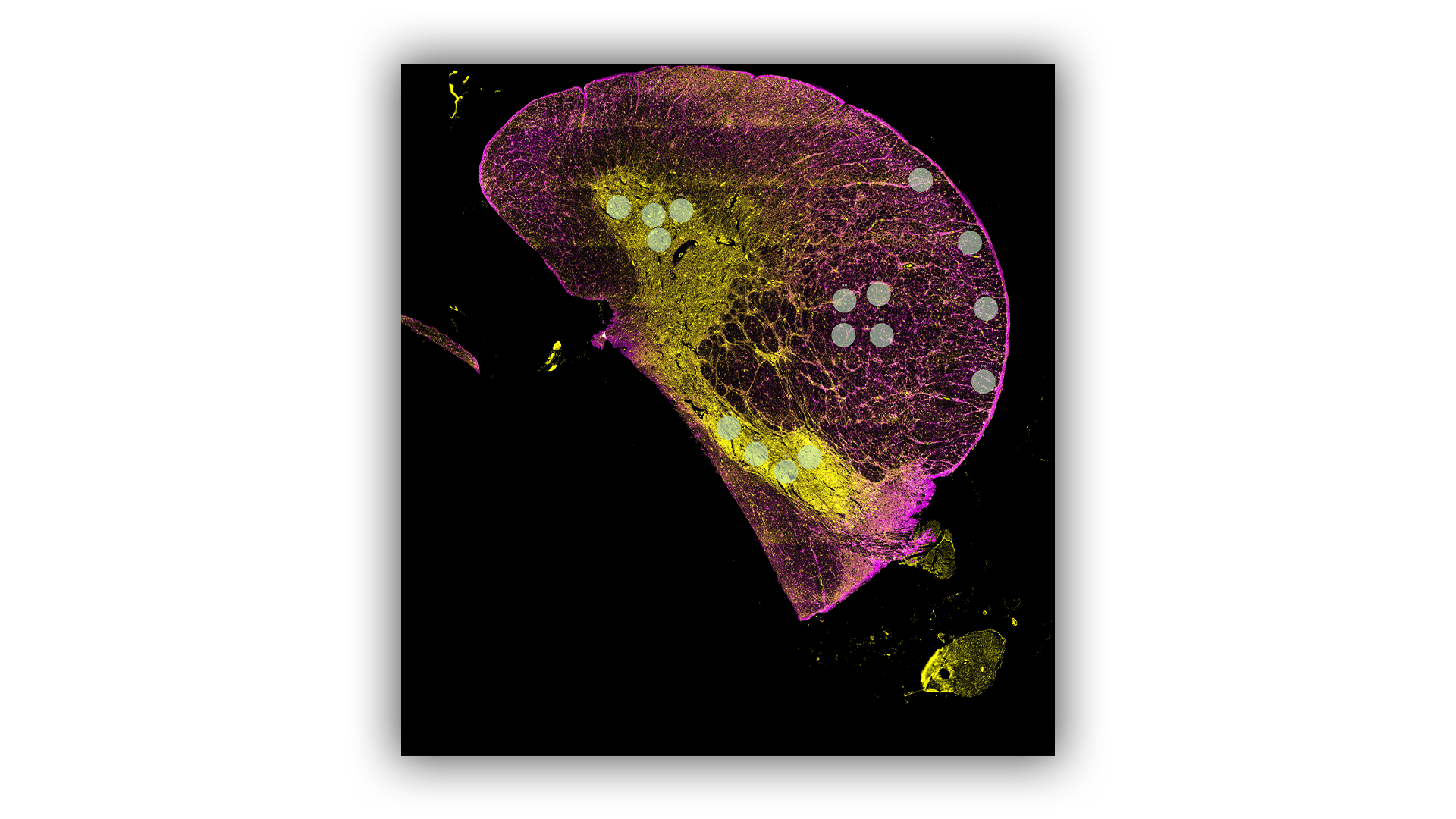
Spatial Proteomics
The spatial localisation of proteins is routinely examined on sectioned tissue using the GeoMx Digital Spatial Profiler (DSP) or Mass Spectrometry Imaging (MSI) platforms. The DSP is used for targeted protein analysis whilst MSI can examine targeted or untargeted proteins.
GeoMx Digital Spatial Profiler
-
- Multiplexed Protein Panels consisting of up to 570 proteins are used to individually profile the proteins in selected regions of interest across a tissue.
- Data can be further segmented for the specific cell types in the regions like neurons, microglia and astrocytes.
- Validated panels include many proteins linked to neurodegenerative diseases, such as NF-L, GFAP, S100B, TDP-43, Amyloid-Beta, Tau, and Alpha-Synuclein.
- If your protein of interest is not already included, we have experience in validating novel targets for inclusion into the multiplex panels.
Mass Spectrometry Imaging
-
-
MSI enables detailed visualisation of the spatial distribution of targeted or untargeted proteins/peptides.
-
It can detect and quantify several protein species at the same time, enhancing the efficiency of proteomic studies.
-
Spatial Transcriptomics
MDC offers several spatial transcriptomics protocols to enable the profiling of spatially localised gene expression patterns across a tissue section using either the DSP-Transcriptomics or SlideSeq platforms. We can discuss which method may be more appropriate depending on the specific research question and budget.
GeoMx Digital Spatial Profiler
-
- Selected regions are profiled for the whole transcriptome of protein coding genes.
- Human, mouse and canine specific codesets available.
Slideseq
-
- Examines up to a 10mm x 10mm area for the whole transcriptome with single cell resolution.
- Species agnostic.
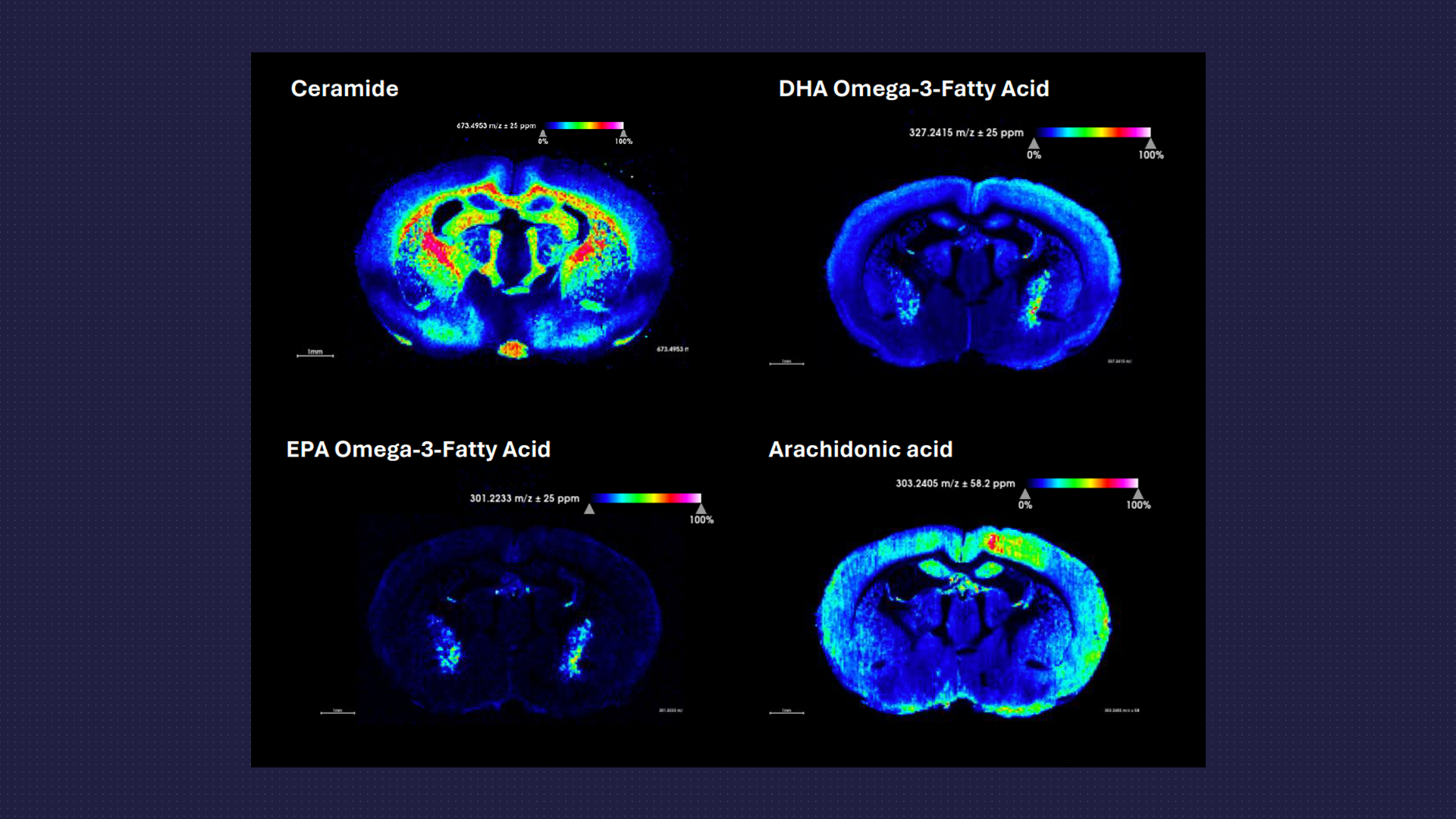
Spatial Lipidomics and Metabolomics
- Using the MSI platform targeted or untargeted analysis of a range of lipids or metabolites can be examined and the spatial resolution across the tissue determined.
- Metabolic dysfunction and neuroinflammation are often intricately linked with neurodegenerative disorders.
- Changes in metabolites and various fatty acid species and their localisation within brain or spinal cord tissue can aid in the understanding of disease processes and specific drug effects.
Sample Acquisition
Whether you’re providing your own samples or need support in obtaining relevant material, we’ve got you covered. Our expertise includes all aspects of acquiring relevant human samples and conducting in vivo models according to your specific requirements.
Human Samples
-
- A dedicated samples team has established connections with various sample providers including biobanks with global networks enabling access to ‘hard to find’ samples at competitive rates.
- They have extensive experience in sourcing a variety of high-quality samples, including plasma and CSF.
- Our samples team is well versed in managing applications for external ethical approvals to ensure compliance with HTA guidelines.
Samples from in vivo Models
-
- In addition to validating new models tailored to your specific research question, the team have expertise in neurosurgical techniques and applying various imaging modalities for monitoring disease progression in the following CNS models:
-
-
- Alzheimer’s Disease (5xFAD model; TG4510)
- Huntington’s Disease (R6/1 model)
- Amyotrophic Lateral Sclerosis (SOD1G93A)
- Orthotopic mouse brain tumour model (U87-Luc; G261-Luc)
- LPS induced model of acute neuroinflammation
-
Reshaping Medicines Discovery...
It's ambitious, it's achievable
-
The collaborative partnership with MDC has enabled us to work together to secure multiple Innovate UK grants. MDC has supported Alchemab in developing a therapeutic for Huntington’s Disease through antibody characterisation, iPSC model development, biodistribution studies, innovative imaging and their general neurodegenerative expertise. The partnership has allowed us to utilise capabilities at MDC, such as state-of-the-art PET imaging abilities, which aren’t available elsewhere.
Alchemab Therapeutics
Our Experts

Gayle Marshall
Head of Biomarkers

Dr Kerry Shea
Lead Scientist

Dr Irma O’Meara
Senior Scientist

Eleanor Platt
Molecular Scientist
Speak to Us
We can help support you with your next drug discovery project.