Biomarkers improve our understanding of drugs, disease, and drug-target interactions within patients, and are key to unlocking a better understanding of:
- Translating drugs from pre-clinical into a clinical setting
- Dose and schedule of treatment that is required to deliver patient benefit
- Morphology changes due to modulation of the target
- Biomarker-based patient selection
- Beneficial combination treatment options
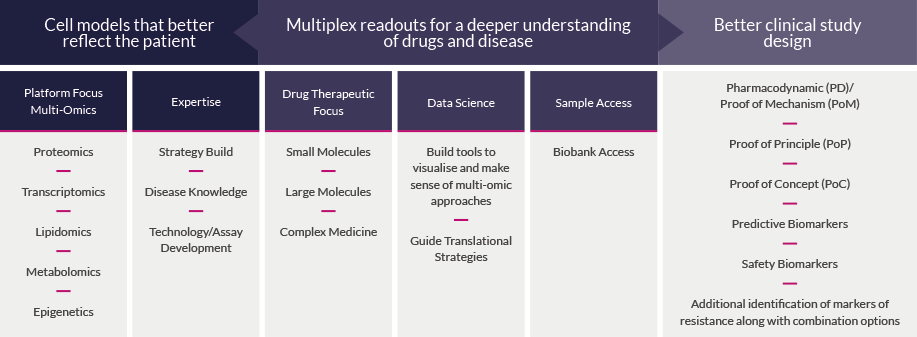
We have established a unique suite of high-specification multi-omic technologies for biomarker discovery and validation, including advanced tissue analysis methods with high spatial resolution, positioned alongside expert bioinformatic analysis. We use the right techniques to address key questions about your projects, improve the validation of innovative projects to increase investability, and help to shape plans for in vivo and clinical testing.
Our Biomarker platform enables you to access complex specialist analytical methods for measuring large numbers of analytes from the same high-value samples. We also provide integrated data sets across different technologies to support your biomarker identification and validation, to progress your compound through the drug discovery process.
Our Biomarker team offers the drug discovery community targeted and bespoke solutions that use highly multiplexed measurement technologies. Combined with expert industry-experienced scientists, we present insightful answers to advance therapeutic opportunities through development. Whatever your challenge we will work with you to develop a bespoke solution.
Through our technical capabilities, expertise and drug discovery know-how, we champion UK innovators to reshape drug discovery for patient benefit.
Did You Know?
An overall drug development failure rate of over 96%, including a 90% failure rate during clinical development, highlights the extent of the challenge.
Clinical trials are expensive, with high attrition rates usually attributed to lack of efficacy, the wrong safety profile, incorrect strategy or poor pharmacokinetics and pharmacodynamics (PK/PD) – an alternative dose-effect analysis used to measure drug concentration in a body compartment.
The rate of drug attrition can be reduced by better understanding the clinical questions and building testable and scientific evidence to transition between pre-clinical and clinical settings.
Biomarker analysis enables a deeper understanding of drugs and disease to ensure better translation into the clinic. To turn molecules into medicines, 5 key areas need to be understood:
- Identify the right clinical questions
- Develop the right biomarker strategy
- Design the right biological assay
- Support the right study design
- Validate using the right clinical samples
A huge number of biomarkers have been identified but very few have entered the clinic as diagnostic tools and many diseases are yet to adopt biomarkers to support and inform the translation of drugs into the clinic.
We can support researchers by identifying the ‘Line of Sight’ for each target through to the clinic and beyond.
- Translational research, identifying the right biomarkers for target engagement and proof of principle in the clinic
- Deep target analysis through the application of bioinformatics
- Identification of new targets in patient samples
- Access to the right human clinical samples to enable disease positioning and disease biology understanding
- Investigation of patient selection strategies, to understand patient subtypes, mechanism of resistance and potential combination options for each program
- Application of a unique suite of molecular biomarker technologies
- Understand current standard of care therapies to ensure approach is compared and differentiated
- Validation of potential diagnostic tools
Our translational capability is based on using the best technology to answer the key translational questions in drug development. We offer bespoke solutions and have a range of advanced multi-modal technologies along with access to high quality samples to validate drug discovery hypothesis and line of sight into the clinic.
We can provide strategic drug discovery and technology platform expertise for solid and liquid tissue across a range of diseases such as Oncology, Fibrosis and Neurodegeneration.

Gayle Marshall
Head of BiomarkersChallenges We Can Help With
Our expertise in biomarkers will allow you to:
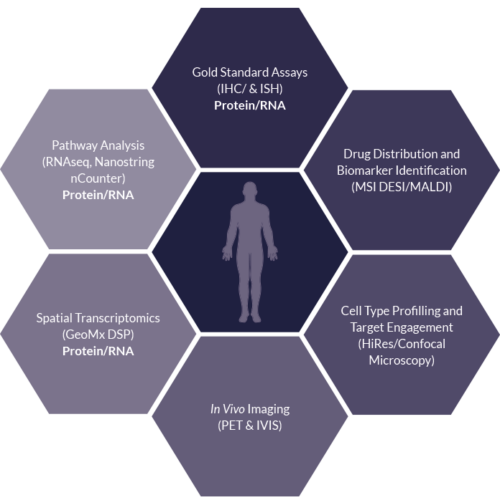
Solid Tissue
Sector Challenge: Deeper understanding of drug and disease – Tissue
- Advancing Biomarker Discovery and validation through combining standard and emerging technologies
- Highly multiplexed readouts can be interrogated for a deeper understanding of drug and disease
- Hypothesis-free and hypothesis-driven biomarker analysis workflows to compare gold standard methods to emerging multiplexing platforms
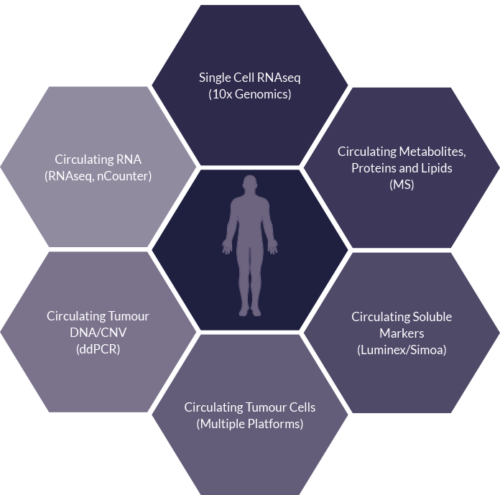
Liquid Tissue
Sector Challenge: Deeper understanding of drug and disease – Liquid Tissue
- Liquid biopsies are just as valuable as they give a real time insight into the patient’s disease and response
- Opportunity to utilise longitudinally
- Additional multiplexed readouts can be interrogated/overlaid to support hypothesis observed
- Circulating factors can provide information on the heterogeneity of the disease along with early response or resistance biomarkers, often before clinical responses are observed
Through Collaborative R&D, we can:
- Enable drug discovery companies to incorporate relevant biomarkers into their programmes, allowing better decision making and greater success in the clinic
- Establish robust methods to analyse large numbers of analytes in small sample volumes with the highest sensitivity
- Provide integrated data sets across different ‘omics technologies and informatics analysis to provide expert interpretation of these studies
- Develop biomarkers from ’omics experiments into clinically relevant assays
Reshaping Medicines Discovery...
It's ambitious, it's achievable
-
By tapping into MDC’s unique drug development expertise and facilities, we are confident we will accelerate the preclinical development of our drug delivery system and its commercialisation.
Dr Anna Perdrix Rosell
Co-founder and Managing Director, Sixfold Bioscience
-
For early-stage SMEs in this space, I thoroughly recommend engaging with MDC who have the end-to-end knowledge in complex medicines, can support your in-house team and provide access to key equipment that start-ups may not have.
Professor Helen McCarthy
Chief Executive Officer, pHion Therapeutics
-
Working with MDC has provided expertise and intellectual input. It has also enabled access to a wider network of companies providing specialist services.
Dr David Templeton
Technical Director, N4 Pharma
-
It has been great to access the Artificial Intelligence expertise at Medicines Discovery Catapult, the team have taken an innovative approach that adds value to our product and will benefit our customers. Our collaboration was easy to establish and worked extremely well.
Phil Jones
CSO, BioAscent
Our Experts

Gayle Marshall
Head of Biomarkers

Dr Bruno Bellina
Lead Scientist

Dr Michael Eyres
Lead Scientist

Eleanor Platt
Molecular Scientist

Dr Lucy Frost
Senior Scientist

Dr Maike Langini
Senior Scientist

Dr Andrzej Rutkowski
Lead Scientist

Dr Kerry Shea
Lead Scientist

Dr Irma O’Meara
Senior Scientist

Regine Anderson
Senior Scientist, Biological Samples

Dr Robert Pedley
Molecular Scientist - Biomarkers

Dr Tara Bowen
Scientist

Mikaela Griffiths
Scientist
Our Capabilities and Technologies
Speak to Us
We can help support you with your next drug discovery project.