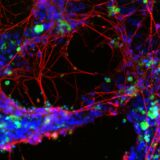
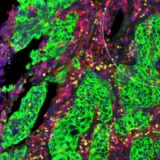
Many neurodegenerative diseases are associated with the presence of misfolded, aggregating proteins within the brain, leading to cytotoxicity and cell death.
The prion hypothesis states that these toxic species spread through the brain via anatomically connected regions, leading to widespread neurodegeneration.
α-synuclein is a protein that is implicated in the pathogenesis of Parkinson’s disease, as well as other synucleinopathies such as dementia with Lewy bodies and multiple system atrophy.
Mutations in the SNCA gene that encodes α-synuclein are known causes of familial, early-onset Parkinson’s disease and are thought to change the structure of the protein to increase beta-sheet formation2 – promoting the misfolding, aggregation and the prion-like spread of α-synuclein pathology through the brain.
View the poster here: