This is a guest post by Claire Patterson at Seda Pharmaceutical Development Services.
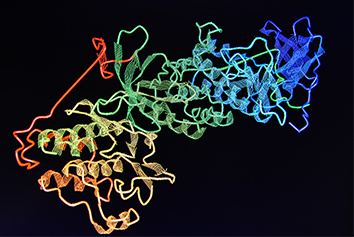
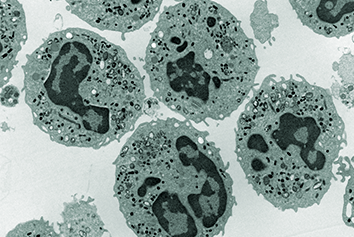
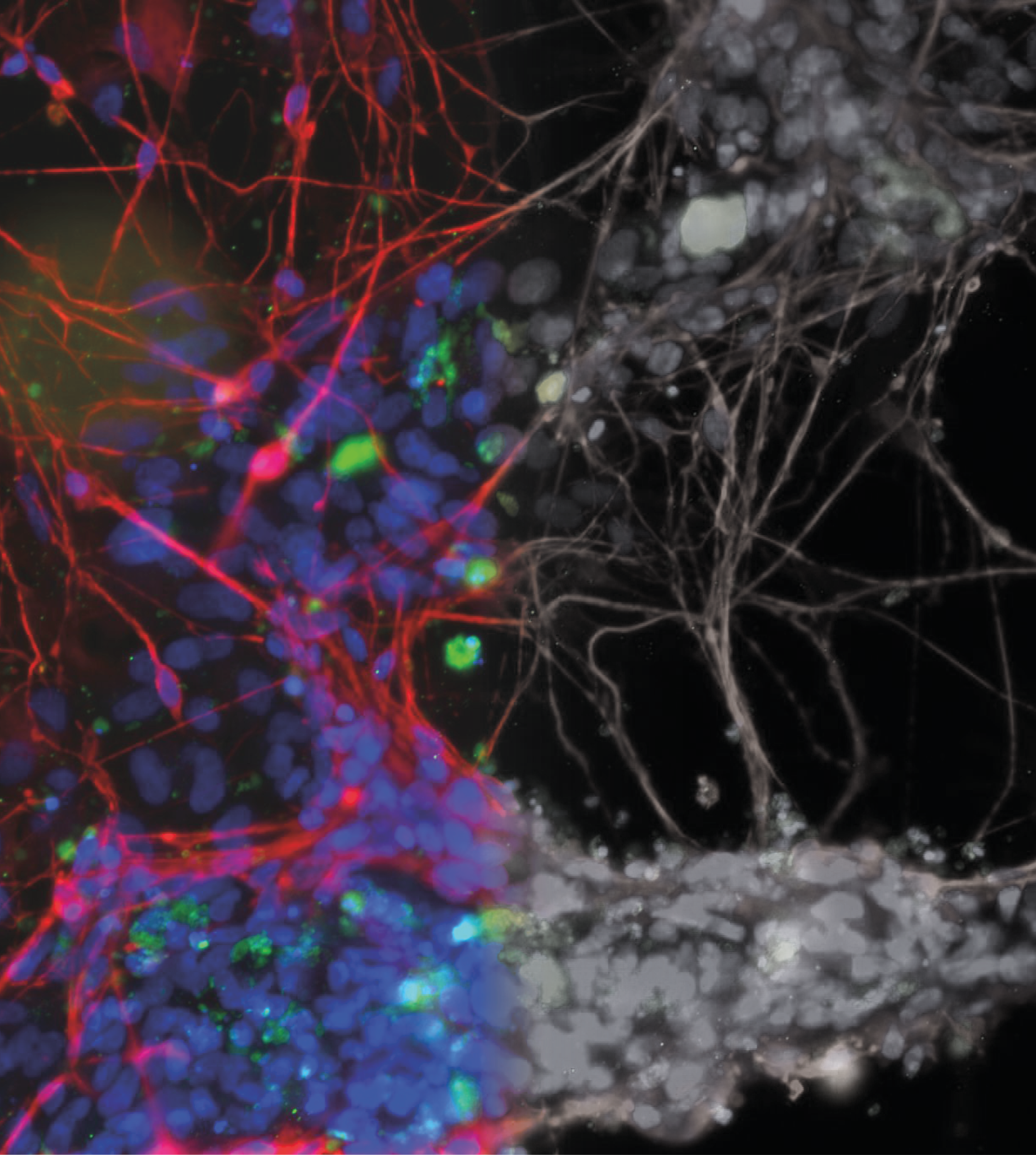
In recent decades there has been a huge increase in the diversification of drug targets and active moieties (synthetic molecules, biologics, and combinations such as antibody drug conjugates) within the drug development field which has led to the development of new, complex medicines to meet patients’ needs. Scientific advances and use of bio- and nanotechnologies has further supported the growing complexity of new medicines.
Whilst no formal, consensus definition of complex medicines exists, the term has evolved to encompass a broad range of medicines that are challenging to characterise. It would typically include all biologics such as vaccines, gene therapies, and recombinant proteins, and a subset of synthetic molecules termed non-biological complex drugs (NBCDs) or complex products. Consensus definitions have not been agreed and terminology and regulatory approval pathway varies across different territories.
From the regulators’ broad umbrella view of complex drug products (incorporating biologicals, NBCDs and hybrids of the two), the Medicines Discovery Catapult postulates that the specific technologies that would benefit from innovation and support include but are not limited to any biological or non-biological drug that falls under one or more of these categories:
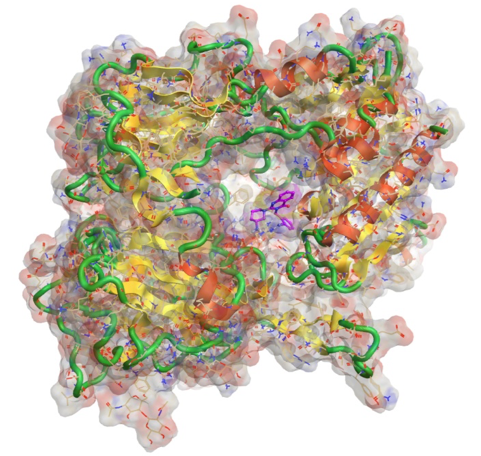
Products with complex active ingredients (e.g., Drug-dendrimer conjugates, glatiramoids, polymeric compounds, antibody drug conjugates, oligonucleotide conjugates, GalNAc-siRNA conjugates).
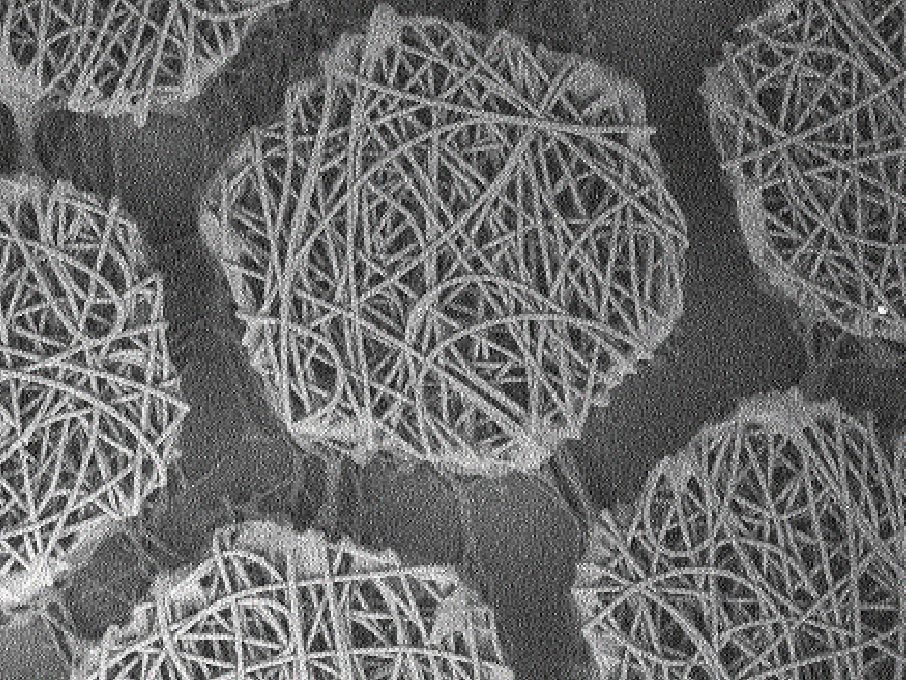
Complex dosage forms/formulations (e.g., Nanomedicines such as liposomes, polymeric/solid-lipid/inorganic nanoparticles, polymersomes, micelles, nanocrystals, colloids, microbubbles, other carriers (chitosan-based carriers for example), albumin-bound agents, extracellular vesicles (exosomes, microvesicles), extended release injectables.
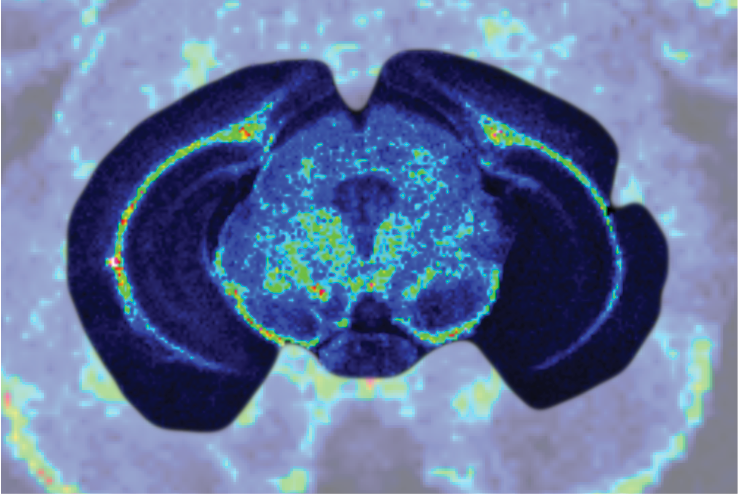
Complex routes of delivery (e.g., Products with a non-systemic site of delivery, including intratumoural, targeted therapies).
Complex medicines can present challenges for innovators and regulatory authorities alike, with established regulatory frameworks having to evolve to accommodate ever diversifying complex medicines.
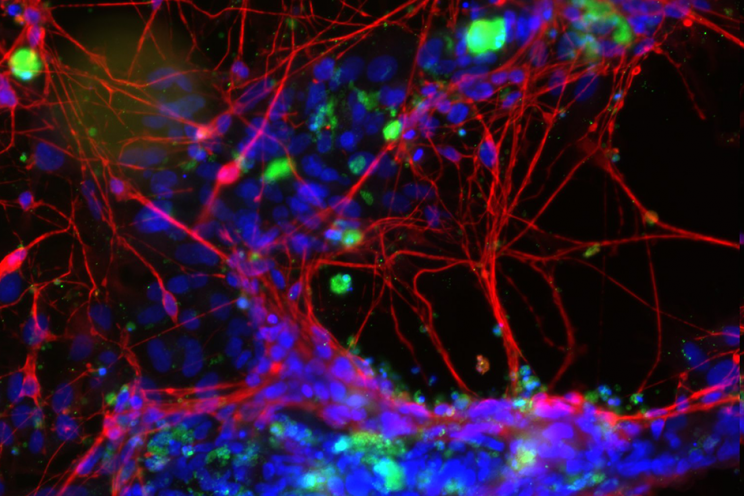
Complex medicines can be challenging to characterise as they often consist of different but closely related structures rather than a single well defined chemical entity, and it can be difficult to identify specific characteristics of the active moiety or its delivery system that might impact the therapeutic performance.
Helping innovators to develop a series of insightful, often novel characterisation techniques to understand the attributes that impact the safety and efficacy of the complex product is a key function of the Medicines Discovery Catapult. These techniques should help innovators develop and manufacture a product of appropriate quality to enable the generation of robust and reproducible preclinical data and, furthermore, to meet drug product quality specifications appropriate to the phase of development (e.g. for first in human clinical studies). Alternatively, the goal may be to generate a data package appropriate to support a claim of similarity to an already marketed product for ‘generics’ manufacturers. The overall aim being to accelerate the development of complex medicines and ensure timely patient access.
We have worked with SEDA Pharmaceutical Development Services to bring clarity to the scope of the term ‘complex medicines’. Download the report to find out more.