Charnwood Molecular is a chemistry CRO, providing medicinal and synthetic chemistry services to the pharmaceutical, biotechnology and chemical industries. Charnwood Molecular support client projects from hit identification through the preclinical development and non GMP toxicity studies.
Synthetic chemistry in drug discovery
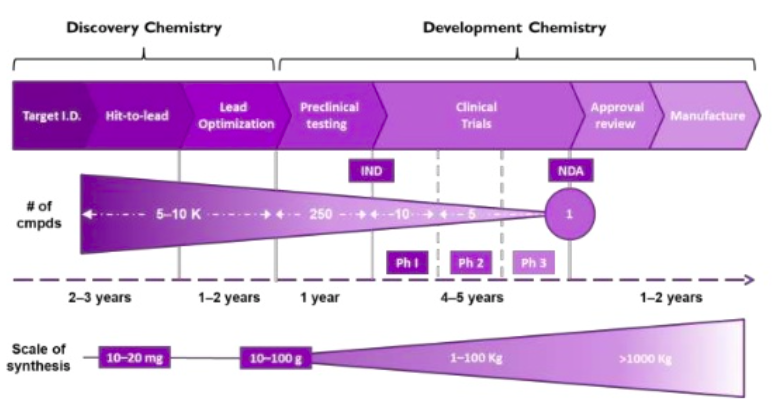
The drug discovery process is complex and scientists collaborating across a variety of different disciplines and areas of expertise to go from identification and validation of a new target, to an approved and marketed small molecule therapeutic agent. Chemistry is fundamental to the drug discovery process with discovery chemists at the front end of the process and development chemists in the latter stages.
Specific skills are required of the chemist to successfully navigate the earlier and latter stages of the drug discovery process, and the two disciplines have apparent differences.
Discovery/medicinal chemists
- Design, synthesise and purify small libraries of compounds
- Use screening cascades to establish structure activity or property relationship
- Understand target biology to ensure desired therapeutic effects are achieved and DMPK liabilities are considered
- Uses computational modelling to generate new ideas for synthesis.
Development chemists
- Focus specifically on the synthesis of a small number of compounds nominated as candidates at the end of a lead optimisation campaign
- Design robust, highly efficient, and cost-effective routes to the candidate compounds
- Require mechanistic understanding of each stage in this synthetic scheme
- Can identify impurities formed during the reaction and to design them out of the process
- Understand the regulatory framework and chemical engineering process to better facilitate the transfer to the pilot plants and beyond.
Use of transformation maps
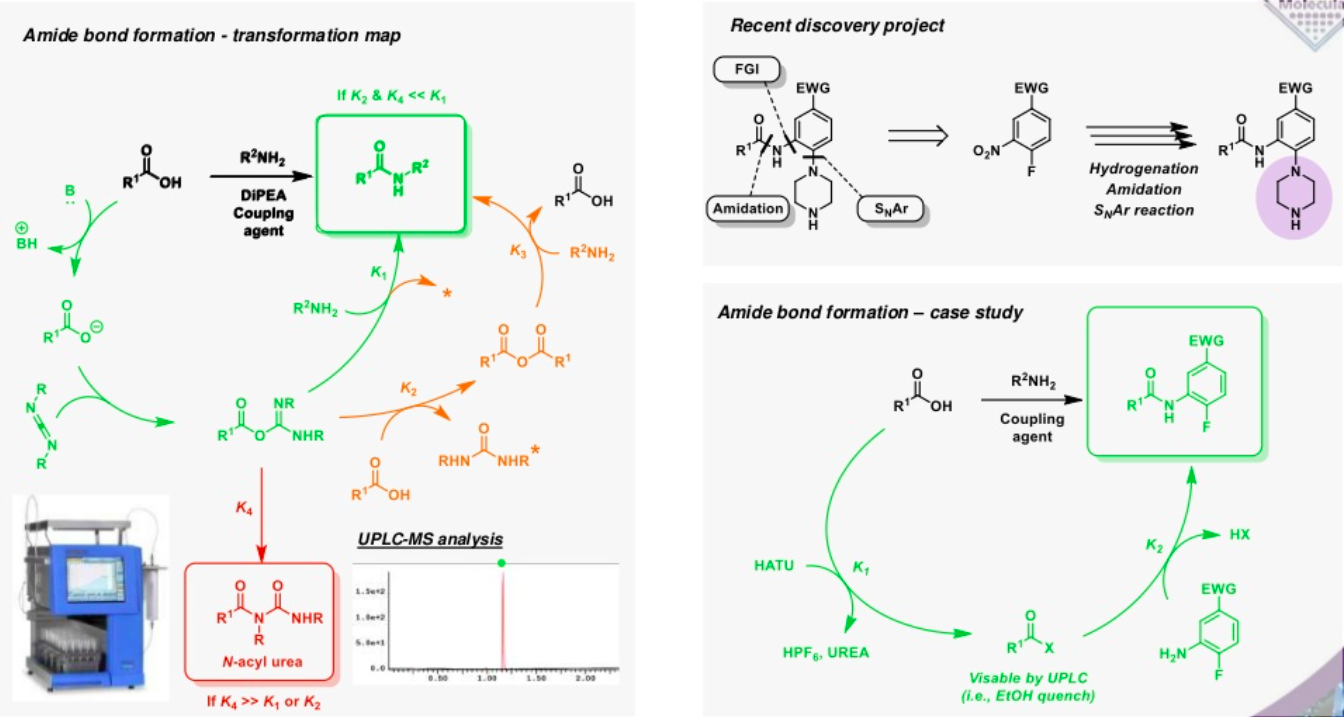
Transformation maps can be applied at any stage in the drug discovery process to provide an in depth understanding of the different pathways available to the many components of the reaction beyond the desired transformation. Understanding and minimising the rate factors driving the different divisionary pathways ultimately facilitates the optimisation of the transformation.
A transformation map can be used to highlight processes that represent the desired transformations (green), semi desirable pathways that have the potential to undermine yield (amber) and deleterious pathways (red) that are of no benefit to the reaction.
For example, unwanted products may form via the rearrangement of an intermediate or its reaction with the starting material or another by-product that may otherwise be considered benign. Understanding these processes can provide a rationale for a low yield, allowing for a solution to be devised, rather than simply purifying the material by column chromatography. In some cases, however, diversionary pathways can also generate useful materials that could appear problematic at first sight. Understanding the nature of these materials can present opportunities, avoiding the need for purification and allowing crude mixture to be advanced into subsequent steps, such as the construction of a small library of analogues, without undermining the efficiency of the process.
Transformation maps provide a detailed understanding of the potential challenges and opportunities in any reaction and provide the potential to deliver considerable savings in time and money.
Further reading
For a more in-depth understanding of synthetic chemistry in the drug discovery process, the following references are recommended:
- Roughley SD and Jordan AM. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J Med Chem 2011;54(10):3451-79
- Brown DG and Boström J. Analysis of past and present synthetic methodologies on medicinal chemistry: Where have all the new reactions gone? J Med Chem 2016;59(10):4443-5
- Brown DG and Boström J. Where do recent small molecule clinical development candidates come from? J Med Chem 2018;61:9442-68
- Beutner GL, et al. TCFH-NMI: Direct access to N-acyl imidazoliums for challenging amide bond formations. Org Lett 2018;20(14):4218-222
This article is based on James’ talk from the MDC Connects webinar series. Watch the session James took part in – Chemistry and Formulation:

About the author
James Hitchin joined Charnwood Molecular in December 2016 as Head of Medicinal Chemistry. James brings extensive experience from across multiple therapeutic areas in drug discovery, having previously held senior positions at SAFC Pharma, Pfizer Pharmaceuticals and KemFine Oy. Prior to joining Charnwood, James was Senior Medicinal Chemist at the Cancer Research UK Manchester Institute, where he worked on various target classes, including kinases and epigenetic targets. James has led numerous projects from hit identification right through to lead optimization and beyond, and has an in depth knowledge of modern drug discovery, as exemplified by his impressive publication record.