Use of preclinical models to deliver proof of concept efficacy
Sygnature Discovery is a leading independent provider of integrated drug discovery and pre-clinical resource and expertise.
Sygnature offer fully integrated discovery project support, as well as discipline-specific support in medicinal and computational chemistry, bioscience, DMPK, in vitro pharmacology, and in vivo pharmacology, as needed by their collaborating clients.
Sygnature’s primary focus is value creation for clients – providing advanced scientific knowledge and intellectual input to accelerate customers’ drug discovery projects from target validation and lead optimisation through to pre-clinical candidate
Recently, Sygnature extended its in vivo pharmacology capabilities by acquiring a translational oncology team which offer bespoke, high-quality oncology in vivo pharmacology services in support of drug discovery projects.
The Translational Oncology team provide in depth oncology and drug discovery knowledge and expertise to their clients. They offer an extensive range of disease-relevant subcutaneous and orthotopic mouse xenograft models and have experience of working with a wide range of therapeutic modalities.
In addition to the team’s expertise in therapeutic PK and tolerability, pharmacodynamic, target engagement and efficacy based studies, the team are also skilled at model development. They are able to undertake model development with commercially available models or work with clients to establish models bespoke to their needs.
Key to success
Target validation is the key to a project’s success. As the biological complexity of the system increases i.e. validation in cell lines, to primary cells, to organoids, through to complex in vivo systems and finally human clinical studies, validation becomes more challenging, however, at each stage, positive data validates the target and decreases the risk of the project failing.
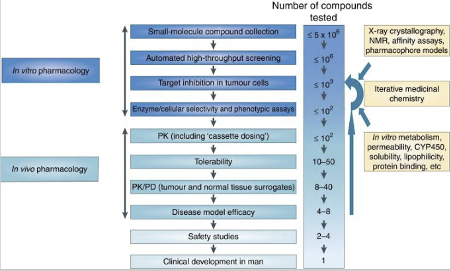
There are several steps required for testing a molecule prior to in vivo, as shown in the drug discovery cascade below. Initial in vitro high throughput screening to identify ‘hits’ involve large numbers of compounds, whereas in in vivo pharmacology, the number of target compounds tested include only compounds with the desired in vitro profile and properties.
There are a number of preclinical murine models available which serve as surrogates for patients including syngeneic models, human cell line derived models, genetically modified models, patient derived explant models and humanised mouse models. The model choice is dictated by the target, the in vitro cell line data and the hypothesis being tested.
Considerations for oncology in vivo proof of concept studies
To ensure a successful in vivo study it is important to consider 3 key elements:
Experimental design:
- Suitable tool molecule for testing (confidence in potency, selectivity, PK)
- Understand PK – dose and schedule (acute and chronic dosing)
- PKPD relationship – not always known as at the start of the in vivo cascades
- Suitable Formulation
- Appropriate controls – vehicles and positive controls
- Monotherapy/combination therapy
- Samples – ensure samples are taken for PK, tumour biomarker assessment and other tissue biomarkers.
Model choice – based on the molecule’s mode of action
- Model should be well characterised – Gene expression, Mutational analysis, Immunophenotype,
- Targeted therapy – cell line derived or patient-derived xenograft models expressing target of interest
- Immune therapy – requires models with a functional immune system e.g. syngeneic models or humanised models
Understand the model of choice
- Understand the utility
- Know how to interpret and use the data
Case studies
Dysregulation of the fibroblast growth factor (FGF)/FGFR pathway is frequently found in many cancer types making this an attractive therapeutic target. AZD4547 is a potent and selective FGFR inhibitor which suppressed FGFR signalling and growth in tumour cell lines with deregulated FGFR. Selection of sensitive and resistant cell line derived xenograft models allowed proof of concept to be tested in vivo. These successful proof of concept pre-clinical studies for AZD4547 have led to phase 2 clinical trials in lung cancer with clinical proof of concept ongoing.
Syngeneic preclinical models can be used to explore proof of concept for immune therapy agents. AZD8835 a dual PI3Ka/d inhibitor was being tested clinically to target tumour epithelial cells in solid tumours. However, the impact of AZD8835 on the tumour microenvironment and anti-tumour immunity had not been explored. Syngeneic models were utilised to investigate the mode of action of AZD8835, these studies revealed a novel immunomodulatory mechanism to deliver anti-tumour activity independent of its effect on tumour cells.
Selecting the right in vivo model to answer the scientific question, to better understand PK, PD, potential biomarkers and establish PK/PD and efficacy relationships is key to validating your target and increasing the probability of success of your project.
This article is based on Lorraine’s talk from the MDC Connects webinar series. Watch the session Lorraine took part in – Target Validation and Efficacy:

About the author
Lorraine Mooney is an experienced Bioscientist with in vivo pharmacology expertise and 15 years’ experience in Oncology Drug Discovery both at AstraZeneca and in contract research. Lorraine has delivered pre-clinical in vivo strategies for large and small molecule drug discovery projects from identifying leads through to supporting early clinical development. Lorraine has expansive knowledge of tumour xenograft models as well as significant experience of utilising syngeneic models to support immune oncology targets. Lorraine received her PhD in Cancer Biology from the University of Sheffield and completed two Postdoctoral Research Positions, studying Stress Kinase Cell Signalling Pathways at the University of Manchester and then at AstraZeneca exploring in vivo optical imaging techniques.