Antiphospholipid syndrome (APS) is an autoimmune disease in which pathogenic autoantibodies cause development of clots, strokes and/or recurrent miscarriages. APS is the main cause of strokes in people under 50 years old and a major cause of pregnancy loss.
Treatment options for APS are very limited. Importantly corticosteroids and immunosuppressive drugs are not effective in reducing either thrombosis risk or rate of miscarriage in patients with APS. In fact, the only evidence-based therapies are long-term anticoagulation for prevention of recurrent thrombosis and heparin and aspirin throughout pregnancy to prevent miscarriage. These treatments are associated with adverse effects, notably risk of haemorrhage.
In recent years, there has been great interest in the possibility of introducing use of direct acting anticoagulants such as rivaroxaban into the management of APS. However, although there were promising results from a British non-inferiority study of warfarin against rivaroxaban in patients with APS, a more recent study in Italy concentrating on APS patients with high thrombosis risk was stopped due to worse outcomes in the rivaroxaban arm. Therefore there is an urgent need for new therapeutic agents in APS.
These agents would be taken long-term by often asymptomatic patients in order to prevent potentially catastrophic outcomes such as ischaemic stroke. Therefore the ideal candidate needs to combine high therapeutic efficacy with low risk of side-effects. Thus we need agents that target the pathogenic process in APS directly.
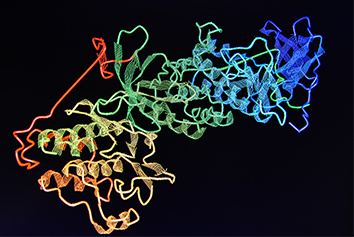
The pathogenic antibodies in patients with APS are called antiphospholipid antibodies (aPL) though this is actually a misnomer, because we now know that the antibodies target phospholipid-binding proteins. The major antigen in APS is beta-2-glycoprotein I (b2GPI), a ubiquitous serum glycoprotein involved in a number of physiological processes, notably coagulation and the complement cascade. A number of potential therapeutics in development target b2GPI, though none has yet reached clinical trials.
The molecule has five domains (I-V). The N-terminal domain (DI) binds the pathogenic aPL and our group at UCL has shown that recombinant DI blocks this binding and reduces thrombosis in a mouse model of APS. Conversely, DV allows the aPL-b2GPI to bind phospholipids on cell surfaces leading to pathogenic effects. Groups in the USA have shown that inhibiting this interaction, either with a peptide derived from cytomegalovirus or a dimer of recombinant domains of the human ApoER2 receptor, can also reduce thrombosis in APS models.
About the author
Professor Anisur Rahman qualified from Oxford in 1988 and trained in rheumatology in London. He obtained his PhD for research into the molecular properties of autoantibodies that cause tissue damage in systemic lupus erythematosus and the antiphospholipid syndrome and began to build up his own research group in 2000 when he was appointed as a Senior Lecturer at University College London. He was awarded the Michael Mason Prize for this research by the British Society of Rheumatology in 2004, and was promoted to a personal chair in rheumatology in 2008.
As well as continuing his basic science research he has developed clinical research programmes in autoimmune rheumatic disease and chronic pain. As a member of both the British Isles Lupus Assessment Group and the Systemic Lupus International Collaborative Clinics he is involved in large multi-centre research projects in the field of lupus.
Professor Rahman’s basic science group is developing a potential new therapeutic agent for the antiphospholipid syndrome.