One of the most important challenges in drug development is efficient delivery of the drug molecule to the appropriate tissue to drive therapeutic benefit, while minimising side effects. For small molecule drugs, a sub-optimal ‘therapeutic index’ between the dose that delivers desired and undesired effects can limit effectiveness or even stop progression through clinical trials. For non-small molecule drugs, such as antibody-drug conjugates and oligonucleotides, the high molecular weight and other properties can limit distribution across the body and therefore prevent the drug from reaching its target and having a therapeutic effect.
The challenge
Imagine an anti-cancer drug designed to kill a tumour. When given to the patient, this drug molecule needs to move through the circulatory system, navigate the extracellular environment, penetrate the tumour tissue before it gets removed and metabolised and finally enter the cell and bind to its target. All while causing minimum effect on normal cells.
The solution
Nanotechnology-based drug delivery systems hold tremendous potential to overcome these challenges. Designed to carry the drug(s) or genes specifically to the target cells or tissues, the systems release it at the desired concentrations, decreasing undesired toxicity effects. This allows for rapid evaluation of treatment efficacy in the early stages.
Over the last decade, dozens of nanomedicines have received clinical approval; with many more in pre-clinical development or in clinical trials.
Critical questions
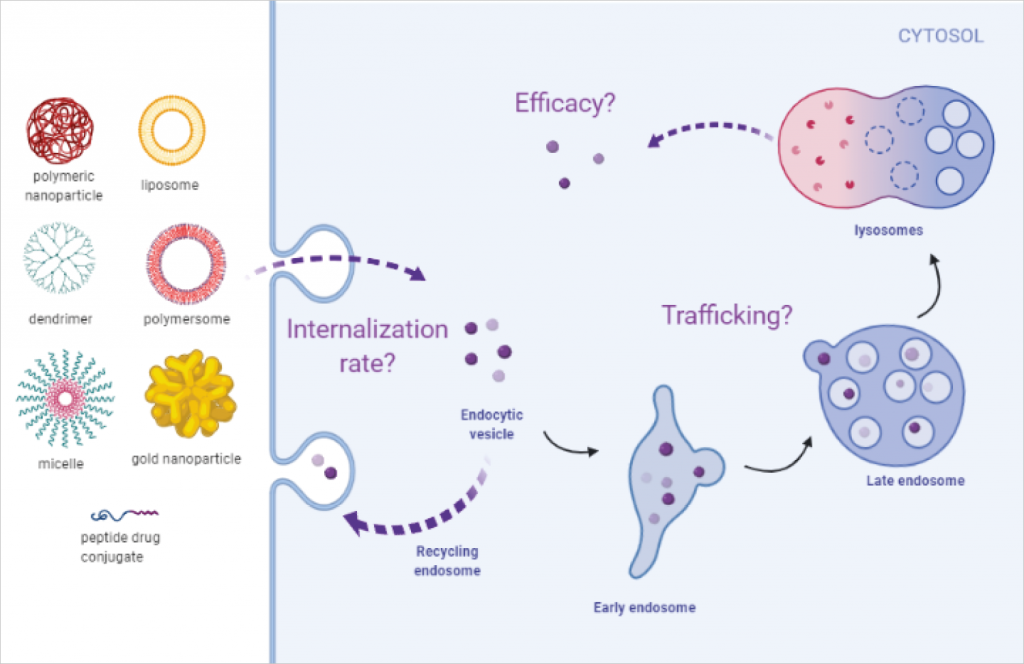
For nanomedicines to be successful in the clinic, in-depth mechanistic understanding of these delivery systems is crucial. There are several critical questions that need answers:
- How do they enter the cell?
- Once they have entered the cell, which cellular compartments do they go to?
- Are they trapped in a specific compartment and degraded?
- Do the cells throw them out before they release their cargo?
- Do they release their cargo at an effective concentration?
Answering these questions will help to develop an effective drug delivery system. Once the mechanism of delivery is understood, properties of the system (size, surface charge, shape, hydrophobicity, surface chemistry) can be tailored to evade the biological barriers and achieve optimal cellular uptake in different organ systems to treat different diseases.
Cellular entry and trafficking of nanomedicines
At Medicines Discovery Catapult, we have developed a microscopy-based platform to study the cellular entry and trafficking of nanomedicines. This image-based platform provides a sensitive and robust method for evaluation of nanomedicines in cellular systems and enables to improve the drug delivery systems further.
About the author
Dr Duygu Yilmaz, PhD, is a Senior Scientist in Target Engagement & Validation at Medicines Discovery Catapult, with over 5 years’ experience in drug research and development industry.
During her PhD, Duygu worked on functionalised liposomal drug delivery systems, which would release the drug payload in a highly sensitive manner in response to stimulus in the environment. She has experience in various other drug delivery systems including polymeric nanoparticles and cell penetrating peptides. At MDC, she is developing microscopy-based methods to study intracellular entry and trafficking of nanoparticles.