Clinical and commercial progress
Nucleic acid therapeutics, which address the underlying causes of disease at the gene and RNA levels, have been on the rise in recent years. CRISPR and mRNA therapies have now entered clinical development, with a major therapeutic area focus on oncology and hematology (gene-editing and mRNA therapies), as well as infectious disease including SARS-CoV-2 (mRNA therapies). Meanwhile, the growing number of approved antisense oligonucleotides and RNAi drugs has also sparked a renewed interest in these modalities.
The initial fanfare around RNAi, including as Science’s “Breakthrough of the Year” in 2002 and the Nobel Prize in Physiology or Medicine in 2006, was relatively short-lived. Late 2000s-early 2010s saw several major pharma players such as Roche, Novartis, Merck, and Pfizer scale back or sell-off their RNAi portfolios given slow clinical progress. However, the much-awaited first FDA approval of Alnylam’s patisiran in 2018 validated the RNAi approach, boosting the wider space.
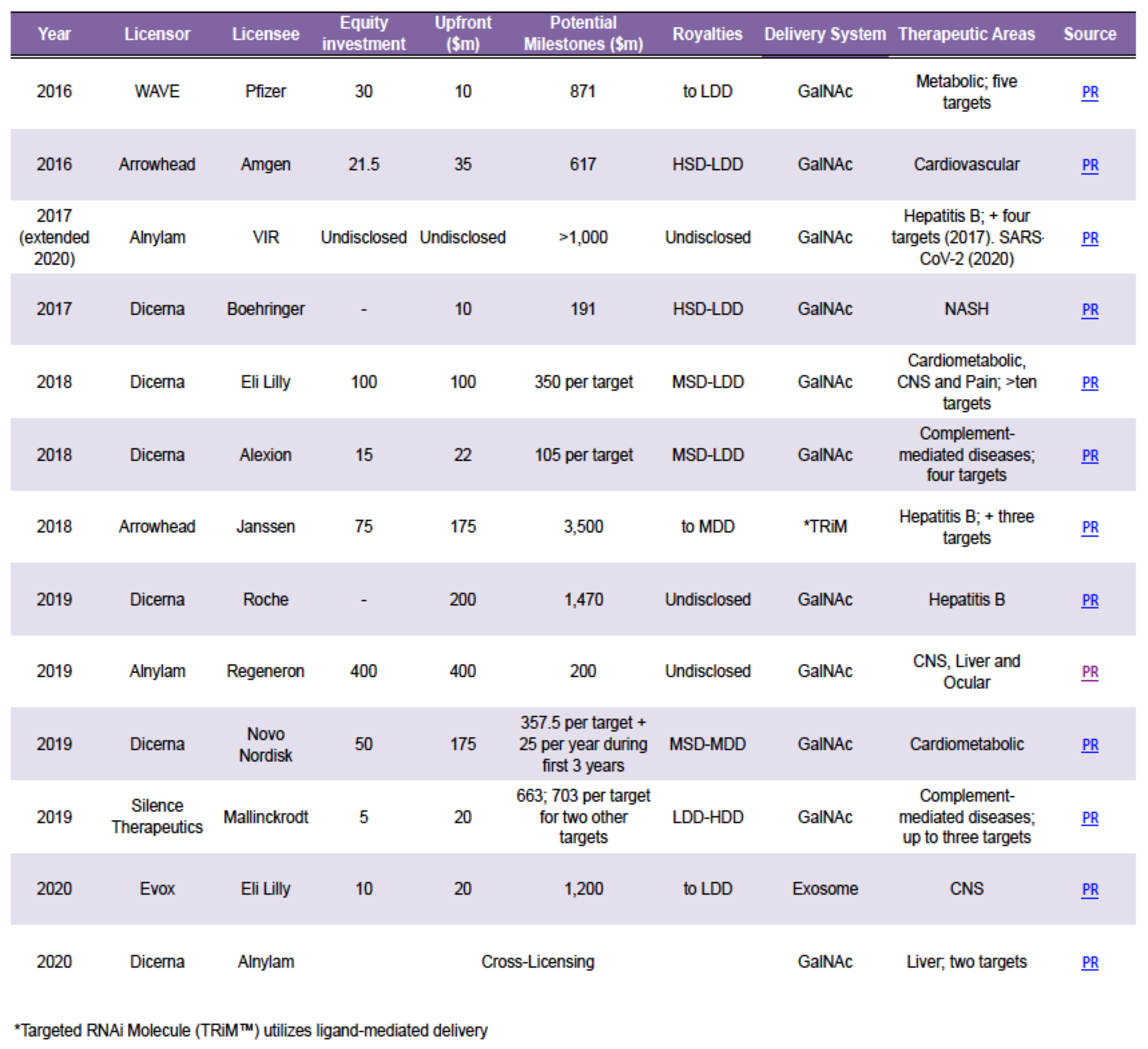
Our recently published analysis showed a clear increase in siRNA clinical activity between 2016-2020. This trend is also reflected in the number of $1bn+ collaboration and licensing deals during the same period (Table 1) and the M&A activity including Novartis’ $9.7bn acquisition of The Medicine Company with its lead siRNA, inclisiran, in 2019.
The Delivery Challenge
However, wide application of nucleic acid therapeutics, including RNAi, depends on effective delivery of these drugs into target tissues, cells and their specific compartments.
Naked nucleic acids are quickly degraded by enzymes in the blood, can evoke immune response, and are too large and negatively charged to passively cross the cell- and other biological membranes. Therefore, their in vivo application relies on vectors capable of transporting them to the desired location whilst overcoming a range of biological barriers. This drug delivery challenge is explained, for example, in the blog post by MDC’s Duygu Yilmaz.
Broadly vehicles for delivery of nucleic acid therapeutics can be divided into viral and non-viral vectors. Viral vectors focus primarily on the use of lentiviruses, whose low cytotoxicity, integration and long-term expression have made them the vector of choice for, e.g. CAR-T cancer therapies; and adeno-associated viruses (AAV), whose structural simplicity, broad applicability and good tolerability have propelled them to be the overall most-widely used platform for gene delivery, including in two approved products AAV1 (uniQure’s Glybera) and AAV2 (Spark Therapeutics’ Luxturna). In the non-viral vector space, two broad categories include encapsulation approaches such as the well-established lipid or lipid-like and polymer nanoparticles as well as exosomes; and perhaps a more conceptually straightforward conjugation methods such as GalNAc for liver delivery, antibody, and cell-penetrating peptides (Figure 1) amongst others, more broadly reviewed in the Nature paper Non-viral vectors for gene-based therapy.
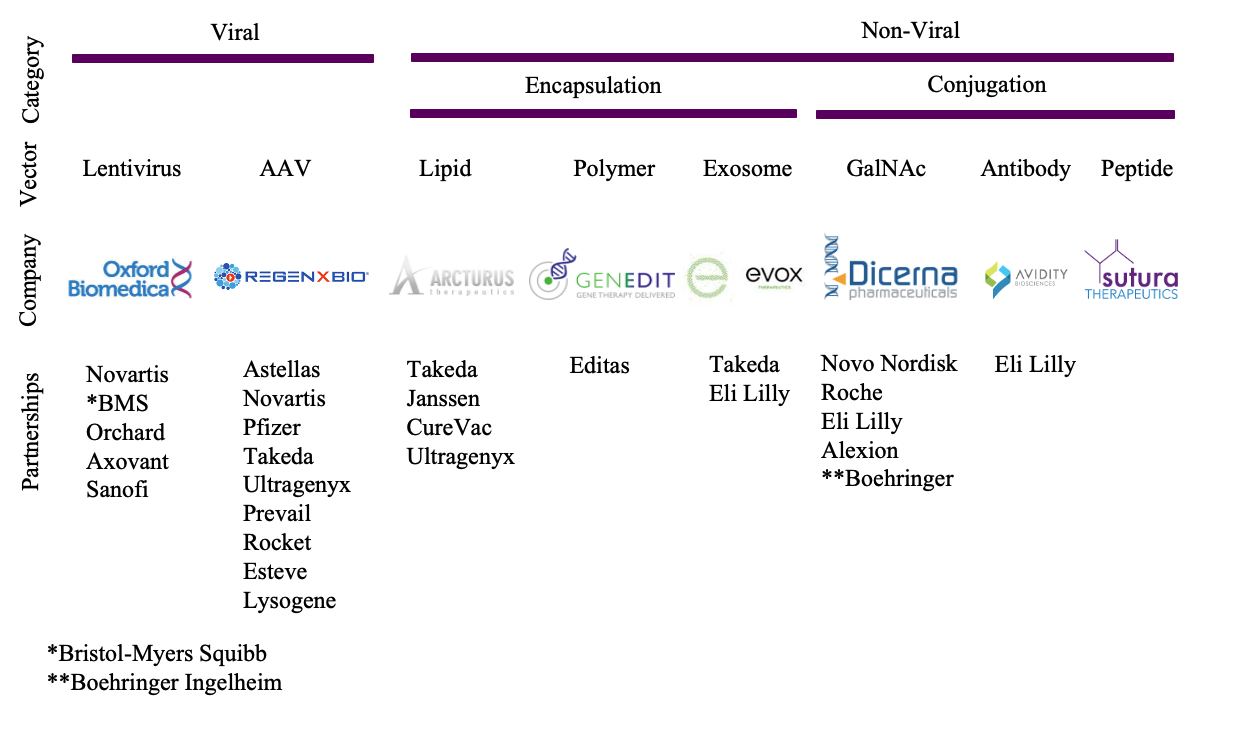
Nucleic acid nanoparticles, such as those developed by Sixfold Bioscience, are an emerging delivery approach. R&D collaborations with MDC that focus on preclinical development of drug delivery systems are important in accelerating a possible translation of novel vectors, such as Sixfold’s, into the clinic.
About the author
Dr Zuzanna Brzosko is the CEO of Sixfold Bioscience, a preclinical-stage biotechnology company focused on developing drug delivery systems for gene therapeutics such as short interfering RNAs for therapeutic gene silencing.
Zuzanna completed her PhD and postdoctoral training at Cambridge University and her undergraduate degree at Oxford University. Following her academic work, she worked in Healthcare Equity Research at Goldman Sachs before founding Sixfold.
Zuzanna has deep interest in the wider healthcare industry and has recently joined the West London NHS Trust as a Non-Executive Director under the Next Director scheme.




















































































































